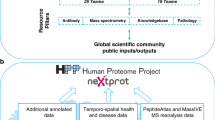
Abstract
The human body contains trillions of cells, classified into specific cell types, with diverse morphologies and functions. In addition, cells of the same type can assume different states within an individual’s body during their lifetime. Understanding the complexities of the proteome in the context of a human organism and its many potential states is a necessary requirement to understanding human biology, but these complexities can neither be predicted from the genome, nor have they been systematically measurable with available technologies. Recent advances in proteomic technology and computational sciences now provide opportunities to investigate the intricate biology of the human body at unprecedented resolution and scale. Here we introduce a big-science endeavour called π-HuB (proteomic navigator of the human body). The aim of the π-HuB project is to (1) generate and harness multimodality proteomic datasets to enhance our understanding of human biology; (2) facilitate disease risk assessment and diagnosis; (3) uncover new drug targets; (4) optimize appropriate therapeutic strategies; and (5) enable intelligent healthcare, thereby ushering in a new era of proteomics-driven phronesis medicine. This ambitious mission will be implemented by an international collaborative force of multidisciplinary research teams worldwide across academic, industrial and government sectors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
23 December 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41586-024-08555-x
References
Venter, J. C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Lander, E. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Aebersold, R. & Mann, M. Mass-spectrometric exploration of proteome structure and function. Nature 537, 347–355 (2016).
Abbott, A. And now for the proteome. Nature 409, 747 (2001).
Fields, S. Proteomics. Proteomics in genomeland. Science 291, 1221–1224 (2001).
The proteome isn’t genome II. Nature 410, 725 (2001).
Adhikari, S. et al. A high-stringency blueprint of the human proteome. Nat. Commun. 11, 5301 (2020).
Omenn, G. S. et al. The 2022 Report on the Human Proteome from the HUPO Human Proteome Project. J. Proteome Res. 22, 1024–1042 (2023).
Kusebauch, U. et al. Human SRMAtlas: a resource of targeted assays to quantify the complete human proteome. Cell 166, 766–778 (2016).
Cyranoski, D. China takes centre stage for liver proteome. Nature 425, 441 (2003).
Jia, H. & Louet, S. China pushes liver proteomics. Nat. Biotechnol. 22, 136 (2004).
He, F. Human liver proteome project: plan, progress, and perspectives. Mol. Cell. Proteomics 4, 1841–1848 (2005).
Wang, J. et al. Toward an understanding of the protein interaction network of the human liver. Mol. Syst. Biol. 7, 536 (2011).
Wang, Q. et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327, 1004–1007 (2010).
Zhao, S. et al. Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004 (2010).
Sharma, K. et al. Cell type- and brain region-resolved mouse brain proteome. Nat. Neurosci. 18, 1819–1831 (2015).
Doll, S. et al. Region and cell-type resolved quantitative proteomic map of the human heart. Nat. Commun. 8, 1469 (2017).
Ni, X. et al. A region-resolved mucosa proteome of the human stomach. Nat. Commun. 10, 39 (2019).
Dyring-Andersen, B. et al. Spatially and cell-type resolved quantitative proteomic atlas of healthy human skin. Nat. Commun. 11, 5587 (2020).
Rieckmann, J. C. et al. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat. Immunol. 18, 583–593 (2017).
Wilhelm, M. et al. Mass-spectrometry-based draft of the human proteome. Nature 509, 582–587 (2014). This paper, together with Kim et al. (2014), shows the initial version of the tissue/organ-centric human proteome by applying mass spectrometry-based approaches.
Kim, M. S. et al. A draft map of the human proteome. Nature 509, 575–581 (2014).
Cyranoski, D. China pushes for the proteome. Nature 467, 380 (2010).
Rodriguez, H., Zenklusen, J. C., Staudt, L. M., Doroshow, J. H. & Lowy, D. R. The next horizon in precision oncology: proteogenomics to inform cancer diagnosis and treatment. Cell 184, 1661–1670 (2021).
Irmisch, A. et al. The Tumor Profiler Study: integrated, multi-omic, functional tumor profiling for clinical decision support. Cancer Cell 39, 288–293 (2021).
Uhlén, M. et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol. Cell. Proteomics. 4, 1920–1932 (2005).
Uhlén, M. et al. Tissue-based map of the human proteome. Science 347, 1260419 (2015).
Tully, B. et al. Addressing the challenges of high-throughput cancer tissue proteomics for clinical application: ProCan. Proteomics 19, e1900109 (2019).
Eldjarn, G. H. et al. Large-scale plasma proteomics comparisons through genetics and disease associations. Nature 622, 348–358 (2023).
Xu, Y. et al. An atlas of genetic scores to predict multi-omic traits. Nature 616, 123–131 (2023).
Sun, B. B. et al. Genomic atlas of the human plasma proteome. Nature 558, 73–79 (2018).
Jiang, Y. et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature 567, 257–261 (2019). This study showcases the concept of proteomics-driven precision medicine.
Kelly, R. T. Single-cell proteomics: progress and prospects. Mol. Cell. Proteomics 19, 1739–1748 (2020).
Mund, A., Brunner, A. D. & Mann, M. Unbiased spatial proteomics with single-cell resolution in tissues. Mol. Cell 82, 2335–2349 (2022). A review summarizing state-of-the-art spatial proteomics for human samples.
Hu, B. C. The human body at cellular resolution: the NIH Human Biomolecular Atlas Program. Nature 574, 187–192 (2019).
Elmentaite, R., Dominguez Conde, C., Yang, L. & Teichmann, S. A. Single-cell atlases: shared and tissue-specific cell types across human organs. Nat. Rev. Genet. 23, 395–410 (2022).
Rozenblatt-Rosen, O. et al. The Human Tumor Atlas Network: charting tumor transitions across space and time at single-cell resolution. Cell 181, 236–249 (2020).
Rajewsky, N. et al. LifeTime and improving European healthcare through cell-based interceptive medicine. Nature 587, 377–386 (2020).
Mann, M., Kumar, C., Zeng, W. F. & Strauss, M. T. Artificial intelligence for proteomics and biomarker discovery. Cell Syst. 12, 759–770 (2021).
Perkel, J. M. Single-cell proteomics takes centre stage. Nature 597, 580–582 (2021).
Guzman, U. H. et al. Ultra-fast label-free quantification and comprehensive proteome coverage with narrow-window data-independent acquisition. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-02099-7 (2024).
MacCoss, M. J. et al. Sampling the proteome by emerging single-molecule and mass spectrometry methods. Nat. Methods 20, 339–346 (2023). A review summarizing next-generation proteomics technologies.
Bi, K. et al. Accurate medium-range global weather forecasting with 3D neural networks. Nature 619, 533–538 (2023).
Davies, A. et al. Advancing mathematics by guiding human intuition with AI. Nature 600, 70–74 (2021).
Thirunavukarasu, A. J. et al. Large language models in medicine. Nat. Med. 29, 1930–1940 (2023).
Kang, M., Ko, E. & Mersha, T. B. A roadmap for multi-omics data integration using deep learning. Brief. Bioinform. 23, bbab454 (2022).
Chen, T. et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 50, D1522–D1527 (2022).
Deutsch, E. W. et al. The ProteomeXchange consortium at 10 years: 2023 update. Nucleic Acids Res. 51, D1539–D1548 (2023).
Fierro-Monti, I., Wright, J. C., Choudhary, J. S. & Vizcaino, J. A. Identifying individuals using proteomics: are we there yet? Front. Mol. Biosci. 9, 1062031 (2022).
Bandeira, N., Deutsch, E. W., Kohlbacher, O., Martens, L. & Vizcaino, J. A. Data management of sensitive human proteomics data: current practices, recommendations, and perspectives for the future. Mol. Cell. Proteomics 20, 100071 (2021).
Deutsch, E. W. et al. Proteomics standards initiative at twenty years: current activities and future work. J. Proteome Res. 22, 287–301 (2023). A perspective paper summarizing the 20-year-long community effort for the proteomics community with respect to data formats, quality control and annotation.
Sharifi-Noghabi, H., Harjandi, P. A., Zolotareva, O., Collins, C. C. & Ester, M. Out-of-distribution generalization from labelled and unlabelled gene expression data for drug response prediction. Nat. Mach. Intell. 3, 962–972 (2021).
Olivella, R. et al. QCloud2: an improved cloud-based quality-control system for mass-spectrometry-based proteomics laboratories. J. Proteome Res. 20, 2010–2013 (2021).
Chawade, A., Alexandersson, E. & Levander, F. Normalyzer: a tool for rapid evaluation of normalization methods for omics data sets. J. Proteome Res. 13, 3114–3120 (2014).
James, F. Monte Carlo theory and practice. Rep. Prog. Phys. 43, 1145 (1980).
Shapiro, E., Biezuner, T. & Linnarsson, S. Single-cell sequencing-based technologies will revolutionize whole-organism science. Nat. Rev. Genet. 14, 618–630 (2013).
Goncalves, E. et al. Pan-cancer proteomic map of 949 human cell lines. Cancer Cell 40, 835–849 (2022).
Qiao, C. et al. Evaluation and development of deep neural networks for image super-resolution in optical microscopy. Nat. Methods 18, 194–202 (2021).
Qiao, C. et al. Rationalized deep learning super-resolution microscopy for sustained live imaging of rapid subcellular processes. Nat. Biotechnol. 41, 367–377 (2023).
Liu, Z. et al. Bioorthogonal photocatalytic proximity labeling in primary living samples. Nat. Commun. 15, 2712 (2024).
Zhang, Z. et al. Progress, challenges and opportunities of NMR and XL-MS for cellular structural biology. JACS Au 4, 369–383 (2024).
Scarmeas, N., Anastasiou, C. A. & Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 17, 1006–1015 (2018).
Kottek, M., Grieser, J., Beck, C., Rudolf, B. & Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 15, 259–263 (2006).
Harel, M. et al. Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell 179, 236–250 (2019).
Xu, J. Y. et al. Integrative proteomic characterization of human lung adenocarcinoma. Cell 182, 245–261 (2020).
Shi, Y. et al. Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature 569, 131–135 (2019).
Eckert, M. A. et al. Proteomics reveals NNMT as a master metabolic regulator of cancer-associated fibroblasts. Nature 569, 723–728 (2019).
Shen, B. et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell 182, 59–72 (2020).
Nie, X. et al. Multi-organ proteomic landscape of COVID-19 autopsies. Cell 184, 775–791 (2021).
Niu, L. et al. Noninvasive proteomic biomarkers for alcohol-related liver disease. Nat. Med. 28, 1277–1287 (2022).
Virreira Winter, S. et al. Urinary proteome profiling for stratifying patients with familial Parkinson’s disease. EMBO Mol. Med. 13, e13257 (2021).
Wigger, L. et al. Multi-omics profiling of living human pancreatic islet donors reveals heterogeneous beta cell trajectories towards type 2 diabetes. Nat. Metab. 3, 1017–1031 (2021).
Johnson, E. C. B. et al. Large-scale deep multi-layer analysis of Alzheimer’s disease brain reveals strong proteomic disease-related changes not observed at the RNA level. Nat. Neurosci. 25, 213–225 (2022).
Zhu, Y. et al. Nanodroplet processing platform for deep and quantitative proteome profiling of 10-100 mammalian cells. Nat. Commun. 9, 882 (2018).
Petelski, A. A. et al. Multiplexed single-cell proteomics using SCoPE2. Nat. Protoc. 16, 5398–5425 (2021).
Su, P. et al. Single cell analysis of proteoforms. J. Proteome Res. 24, 1883–1893 (2024).
Chen, W. et al. Simple and integrated spintip-based technology applied for deep proteome profiling. Anal. Chem. 88, 4864–4871 (2016).
Mund, A. et al. Deep visual proteomics defines single-cell identity and heterogeneity. Nat. Biotechnol. 40, 1231–1240 (2022).
Geyer, P. E. et al. Plasma proteome profiling to assess human health and disease. Cell Syst. 2, 185–195 (2016).
Deutsch, E. W. et al. Advances and utility of the human plasma proteome. J. Proteome Res. 20, 5241–5263 (2021).
Buljan, M. et al. A computational framework for the inference of protein complex remodeling from whole-proteome measurements. Nat. Methods 20, 1523–1529 (2023).
Mackmull, M. T. et al. Global, in situ analysis of the structural proteome in individuals with Parkinson’s disease to identify a new class of biomarker. Nat. Struct. Mol. Biol. 29, 978–989 (2022).
Tsamardinos, I. et al. Just Add Data: automated predictive modeling for knowledge discovery and feature selection. NPJ Precis. Oncol. 6, 38 (2022).
Bai, Y. et al. AutoDC: an automatic machine learning framework for disease classification. Bioinformatics 38, 3415–3421 (2022).
Elmarakeby, H. A. et al. Biologically informed deep neural network for prostate cancer discovery. Nature 598, 348–352 (2021).
Acknowledgements
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (grant no. 2020YFE0202200), the National Natural Science Foundation of China (no. 32088101), National Institutes of Health grants P30ES017885-11-S1 and U24CA271037 (G.S.O.) and the Big-Science Infrastructure of Phronesis Medicine, of which the pilot phase is funded by Guangzhou Development District.
Author information
Authors and Affiliations
Consortia
Contributions
F.H. conceived the concept of π-HuB and designed its scientific goals, and contributed ideas for phronesis medicine with L.X., F.H., R.A., M.S.B., X.W.B., X.C.B., D.W.C., C.C., L.C., X.C., H.C., F.C., W.E., J.F., P.F., D.F., G.F.G., W.G., Z.-H.G., K.G., W.W.B.G., D.G., C.G., T.G., A.J.R.H., H.H., T.H., N.G.I., Y.J., C.R.J., L.J., N.L.K., M.L., Y.L., Q.L., C.H.L., F.L., G.-H.L., Y.S.L., Z.L., T.Y.L., B.L., M.M., A.M., R.L.M., E.N., G.N., G.S.O., G.P., Y.P., C.P., T.C.W.P., A.P., J.Q., R.R., P.J.R., P.R., C.S., J.S., E.S., S.S., A.S., S.K.S., C.T., L.T., R.T., J.V.E., J.A.V., C.W., X.W.W., X.X.W., Y.W., T.W., M.W., R.W., B.W., L.W., L.X., W.X., Tao Xu, L.Y., J.Y., X.Y., J.R.Y., Q.W.Z., L.H.Z., L.Q.Z., Y.K.Z., Q.Z. and Y.P.Z. contributed ideas and suggestions for the conception and design of this project. T.G., L.T. and Y.W. contributed coordination of the π-HuB Consortium. J.Y. wrote the first draft of the manuscript, and created the figures with F.H., T.G., Y.L. and L.X. F.H., R.A., M.S.B., F.C., P.F., D.F., Z.-H.G., K.G., W.W.B.G., T.G., H.H., T.H., N.G.I., C.R.J., L.J., M.L., Q.L., F.L., Y.S.L., T.Y.L., R.L.M., G.S.O., T.C.W.P., A.P., R.R., P.J.R., C.S., S.K.S., J.A.V., T.W., R.W., B.W., L.W., J.Y., J.R.Y. and Q.Z. provided important edits to the manuscript. All authors contributed to review and editing of the manuscript. The π-HuB Consortium contributed to the discussion of strategic π-HuB research plans.
Corresponding authors
Ethics declarations
Competing interests
R.A. holds shares of Biognosys AG, which operates in the field covered by the article. D.F. is co-founder of MedBiome Inc., a precision nutrition company. K.G. is a shareholder of CYBO, LucasLand, and FlyWorks. T.G. is the founder of Westlake Omics Inc. M.M. is an indirect investor in EvoSep. R.T. is a founder of BayOmics. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Tamar Geiger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, F., Aebersold, R., Baker, M.S. et al. π-HuB: the proteomic navigator of the human body. Nature 636, 322–331 (2024). https://doi.org/10.1038/s41586-024-08280-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-08280-5
This article is cited by
-
Mass-spectrometry-based proteomics: from single cells to clinical applications
Nature (2025)
-
Caging AI
Journal of Computer Science and Technology (2025)