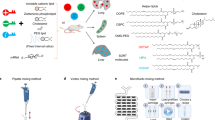
Abstract
Alpha-1 antitrypsin (A1AT) deficiency (AATD) is caused by a mutation in the SERPINA1 gene (PiZ allele), where misfolded A1AT liver accumulation leads to liver damage, and A1AT deficiency in the lungs results in emphysema due to unregulated neutrophil elastase activity. Base editing offers a potential cure for A1AT; however, effective treatment is hindered by the absence of dual-target delivery systems that can target key tissues. We developed Dual Selective ORgan-Targeting lipid nanoparticles (SORT LNPs) to deliver base editors to the liver and lungs. Dual SORT LNPs correct the PiZ mutation, achieving 40% correction editing in liver cells and 10% in lung AT2 cells. The liver maintains stable editing for 32 weeks, reducing Z-A1AT levels by over 80% and restoring a normal liver phenotype. In parallel, 89% neutrophil elastase inhibition is achieved in lung bronchoalveolar lavage fluid. Taken together, Dual SORT LNP therapy offers a promising approach for long-lasting genome correction for multi-organ diseases such as AATD.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data needed to evaluate the conclusions in this paper are present in the main text and the supplementary material. The flow cytometry raw data are available from the corresponding author upon reasonable request.
References
Strnad, P., McElvaney, N. G. & Lomas, D. A. Alpha1-antitrypsin deficiency. N. Engl. J. Med. 382, 1443–1455 (2020).
Tejwani, V. & Stoller, J. K. The spectrum of clinical sequelae associated with alpha-1 antitrypsin deficiency. Ther. Adv. Chronic Dis. 12_suppl:2040622321995691 (2021).
Santos, G. F., Ellis, P., Farrugia, D. & Turner, A. M. Nephrotic syndrome secondary to alpha-1 antitrypsin deficiency. BMJ Case Rep. 14, e240288 (2021).
Loring, H. S. & Flotte, T. R. Current status of gene therapy for α-1 antitrypsin deficiency. Expert Opin. Biol. Ther. 15, 329–336 (2015).
Hamesch, K. & Strnad, P. Non-invasive assessment and management of liver involvement in adults with alpha-1 antitrypsin deficiency. Chronic Obstr. Pulm. Dis. 7, 260–271 (2020).
Cazzola, M., Stolz, D., Rogliani, P. & Matera, M. G. α1-Antitrypsin deficiency and chronic respiratory disorders. Eur. Respir. Rev. 29, 190073 (2020).
Packer, M. S. et al. Evaluation of cytosine base editing and adenine base editing as a potential treatment for alpha-1 antitrypsin deficiency. Mol. Ther. 30, 1396–1406 (2022).
Werder, R. B. et al. Adenine base editing reduces misfolded protein accumulation and toxicity in alpha-1 antitrypsin deficient patient iPSC-hepatocytes. Mol. Ther. 29, 3219–3229 (2021).
Stiles, K. M. et al. Intrapleural gene therapy for alpha-1 antitrypsin deficiency-related lung disease. Chronic Obstr. Pulm. Dis. 5, 244–257 (2018).
Janosz, E. et al. Pulmonary transplantation of alpha-1 antitrypsin (AAT)-transgenic macrophages provides a source of functional human AAT in vivo. Gene Ther. 28, 477–493 (2021).
Chiuchiolo, M. J. & Crystal, R. G. Gene therapy for alpha-1 antitrypsin deficiency lung disease. Ann. Am. Thorac. Soc. 13, S352–S369 (2016).
Raevens, S., Boret, M., De Pauw, M., Fallon, M. B. & Van Vlierberghe, H. Pulmonary abnormalities in liver disease: relevance to transplantation and outcome. Hepatology 74, 1674–1686 (2021).
Zamora, M. R. & Ataya, A. Lung and liver transplantation in patients with alpha-1 antitrypsin deficiency. Ther. Adv. Chronic Dis. 12_suppl:20406223211002988 (2021).
Conrad, A. et al. Impact of alpha 1-antitrypsin deficiency and prior augmentation therapy on patients’ survival after lung transplantation. Eur. Respir. J. 50, 1700962 (2017).
Gulack, B. C. et al. Survival after lung transplantation in recipients with alpha-1-antitrypsin deficiency compared to other forms of chronic obstructive pulmonary disease: a national cohort study. Transpl. Int. 31, 45–55 (2018).
van ‘t Wout, E. F., van Schadewijk, A., Savage, N. D., Stolk, J. & Hiemstra, P. S. α1-Antitrypsin production by proinflammatory and antiinflammatory macrophages and dendritic cells. Am. J. Respir. Cell Mol. Biol. 46, 607–613 (2012).
Belchamber, K. B. R., Walker, E. M., Stockley, R. A. & Sapey, E. Monocytes and macrophages in alpha-1 antitrypsin deficiency. Int. J. Chron. Obstruct. Pulmon. Dis. 15, 3183–3192 (2020).
Hurley, K. et al. Deriving type II alveolar cells from pluripotent stem cells to produce a novel model of alpha-1 antitrypsin deficiency pathogenesis. Eur. Respir. J. 48, PA4659 (2016).
Pini, L. et al. The role of bronchial epithelial cells in the pathogenesis of COPD in Z-alpha-1 antitrypsin deficiency. Respir. Res. 15, 112 (2014).
Abo, K. M. et al. Pulmonary cellular toxicity in alpha-1 antitrypsin deficiency. Chest 166, 472–479 (2024).
Vieira Braga, F. A. et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat. Med. 25, 1153–1163 (2019).
Zhou, K. et al. Modular degradable dendrimers enable small RNAs to extend survival in an aggressive liver cancer model. Proc. Natl Acad. Sci. USA 113, 520–525 (2016).
Dong, Y., Siegwart, D. J. & Anderson, D. G. Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 144, 133–147 (2019).
Han, X. et al. Ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis. Nat. Commun. 14, 75 (2023).
Cheng, Q. et al. Dendrimer-based lipid nanoparticles deliver therapeutic FAH mRNA to normalize liver function and extend survival in a mouse model of hepatorenal tyrosinemia type I. Adv. Mater. 30, e1805308 (2018).
Hou, X. et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 15, 41–46 (2020).
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Alvarez-Benedicto, E. et al. Spleen SORT LNP generated in situ CAR T cells extend survival in a mouse model of lymphoreplete B cell lymphoma. Angew. Chem. Int. Ed. Engl. 62, e202310395 (2023).
Cheng, Q. et al. In situ production and secretion of proteins endow therapeutic benefit against psoriasiform dermatitis and melanoma. Proc. Natl Acad. Sci. USA 120, e2313009120 (2023).
Han, X. et al. Adjuvant lipidoid-substituted lipid nanoparticles augment the immunogenicity of SARS-CoV-2 mRNA vaccines. Nat. Nanotechnol. 18, 1105–1114 (2023).
Xue, L. et al. Combinatorial design of siloxane-incorporated lipid nanoparticles augments intracellular processing for tissue-specific mRNA therapeutic delivery. Nat. Nanotechnol. 20, 132–143 (2024).
Gong, N. Q. et al. Tumour-derived small extracellular vesicles act as a barrier to therapeutic nanoparticle delivery. Nat. Mater. 23, 1736–1747 (2024).
Chaudhary, N. et al. Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d. Nat. Biomed. Eng. 8, 1483–1498 (2024).
Petersen, D. M. S. et al. Branched-tail lipid nanoparticles for intravenous mRNA delivery to lung immune, endothelial, and alveolar cells in mice. Adv. Healthc. Mater. 13, e2400225 (2024).
Mukherjee, A. et al. Engineered mutant α-ENaC subunit mRNA delivered by lipid nanoparticles reduces amiloride currents in cystic fibrosis-based cell and mice models. Sci. Adv. 6, eabc5911 (2020).
Xu, S. F. et al. Tumor-tailored ionizable lipid nanoparticles facilitate IL-12 circular RNA delivery for enhanced lung cancer immunotherapy. Adv. Mater. 36, e2400307 (2024).
Li, B. W. et al. Accelerating ionizable lipid discovery for mRNA delivery using machine learning and combinatorial chemistry. Nat. Mater. 23, 1002–1008 (2024).
Miller, J. B. et al. Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew. Chem. Int. Ed. Engl. 56, 1059–1063 (2017).
Finn, J. D. et al. A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep. 22, 2227–2235 (2018).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Musunuru, K. et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 593, 429–434 (2021).
Tsuchida, C. A., Wasko, K. M., Hamilton, J. R. & Doudna, J. A. Targeted nonviral delivery of genome editors in vivo. Proc. Natl Acad. Sci. USA 121, e2307796121 (2024).
Ely, Z. A. et al. A prime editor mouse to model a broad spectrum of somatic mutations in vivo. Nat. Biotechnol. 42, 424–436 (2024).
Jiang, C. et al. A non-viral CRISPR/Cas9 delivery system for therapeutically targeting HBV DNA and pcsk9 in vivo. Cell Res. 27, 440–443 (2017).
Han, J. P. et al. In vivo delivery of CRISPR–Cas9 using lipid nanoparticles enables antithrombin gene editing for sustainable hemophilia A and B therapy. Sci. Adv. 8, eabj6901 (2022).
Li, B. et al. Combinatorial design of nanoparticles for pulmonary mRNA delivery and genome editing. Nat. Biotechnol. 41, 1410–1415 (2023).
Gao, S. L. et al. Harnessing non-Watson–Crick’s base pairing to enhance CRISPR effectors cleavage activities and enable gene editing in mammalian cells. Proc. Natl Acad. Sci. USA 121, e2308415120 (2024).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Song, C. Q. et al. Adenine base editing in an adult mouse model of tyrosinaemia. Nat. Biomed. Eng. 4, 125–130 (2020).
Rothgangl, T. et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 39, 949–957 (2021).
Ryu, S. M. et al. Adenine base editing in mouse embryos and an adult mouse model of Duchenne muscular dystrophy. Nat. Biotechnol. 36, 536–539 (2018).
Lee, S. M. et al. A systematic study of unsaturation in lipid nanoparticles leads to improved mRNA transfection in vivo. Angew. Chem. Int. Ed. Engl. 60, 5848–5853 (2021).
Wang, X. et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023).
Wei, T. et al. Lung SORT LNPs enable precise homology-directed repair mediated CRISPR/Cas genome correction in cystic fibrosis models. Nat. Commun. 14, 7322 (2023).
Shepherd, S. J., Issadore, D. & Mitchell, M. J. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials 274, 120826 (2021).
van ‘t Wou, E. F. et al. Increased ERK signalling promotes inflammatory signalling in primary airway epithelial cells expressing Z α1-antitrypsin. Hum. Mol. Genet. 23, 929–941 (2014).
Richter, M. F. et al. Phage-assisted evolution of an adenine base editor with improved Cas ___domain compatibility and activity. Nat. Biotechnol. 38, 883–891 (2020).
Abbasi, S. et al. Co-encapsulation of Cas9 mRNA and guide RNA in polyplex micelles enables genome editing in mouse brain. J. Control. Release 332, 260–268 (2021).
Schmidheini, L. et al. Continuous directed evolution of a compact CjCas9 variant with broad PAM compatibility. Nat. Chem. Biol. 20, 333–343 (2024).
Hoseini, B. et al. Application of ensemble machine learning approach to assess the factors affecting size and polydispersity index of liposomal nanoparticles. Sci. Rep. 13, 18012 (2023).
Danaei, M. et al. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 10, 57 (2018).
North, T. L. et al. A study of common Mendelian disease carriers across ageing British cohorts: meta-analyses reveal heterozygosity for alpha 1-antitrypsin deficiency increases respiratory capacity and height. J. Med. Genet. 53, 280–288 (2016).
Shen, S. et al. Amelioration of alpha-1 antitrypsin deficiency diseases with genome editing in transgenic mice. Hum. Gene Ther. 29, 861–873 (2018).
Carlson, J. A. et al. Accumulation of PiZ alpha 1-antitrypsin causes liver damage in transgenic mice. J. Clin. Invest. 83, 1183–1190 (1989).
Bjursell, M. et al. Therapeutic genome editing with CRISPR/Cas9 in a humanized mouse model ameliorates α1-antitrypsin deficiency phenotype. EBioMedicine 29, 104–111 (2018).
Mitchell, E. L. & Khan, Z. Liver disease in alpha-1 antitrypsin deficiency: current approaches and future directions. Curr. Pathobiol. Rep. 5, 243–252 (2017).
Santos, G. & Turner, A. M. Alpha-1 antitrypsin deficiency: an update on clinical aspects of diagnosis and management. Fac. Rev. 9, 1 (2020).
Piccolo, P. et al. Down-regulation of hepatocyte nuclear factor-4α and defective zonation in livers expressing mutant Z α1-antitrypsin. Hepatology 66, 124–135 (2017).
Mela, M. et al. The alpha-1 antitrypsin polymer load correlates with hepatocyte senescence, fibrosis stage and liver-related mortality. Chronic Obstr. Pulm. Dis. 7, 151–162 (2020).
Strnad, P. et al. Fazirsiran for liver disease associated with alpha1-antitrypsin deficiency. N. Engl. J. Med. 387, 514–524 (2022).
Zorzetto, M. et al. SERPINA1 gene variants in individuals from the general population with reduced α1-antitrypsin concentrations. Clin. Chem. 54, 1331–1338 (2008).
Chen, K. et al. Lung and liver editing by lipid nanoparticle delivery of a stable CRISPR–Cas9 ribonucleoprotein. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02437-3 (2024).
Xue, L. et al. Combinatorial design of siloxane-incorporated lipid nanoparticles augments intracellular processing for tissue-specific mRNA therapeutic delivery. Nat. Nanotechnol. 20, 132–143 (2025).
Karadagi, A. et al. Systemic modified messenger RNA for replacement therapy in alpha 1-antitrypsin deficiency. Sci. Rep. 10, 7052 (2020).
Dilliard, S. A. et al. The interplay of quaternary ammonium lipid structure and protein corona on lung-specific mRNA delivery by selective organ targeting (SORT) nanoparticles. J. Control. Release 361, 361–372 (2023).
Takahashi, Y., Nishikawa, M., Takiguchi, N., Suehara, T. & Takakura, Y. Saturation of transgene protein synthesis from mRNA in cells producing a large number of transgene mRNA. Biotechnol. Bioeng. 108, 2380–2389 (2011).
Ogushi, F., Fells, G. A., Hubbard, R. C., Straus, S. D. & Crystal, R. G. Z-type alpha 1-antitrypsin is less competent than M1-type alpha 1-antitrypsin as an inhibitor of neutrophil elastase. J. Clin. Invest. 80, 1366–1374 (1987).
Molloy, K. et al. Clarification of the risk of chronic obstructive pulmonary disease in α1-antitrypsin deficiency PiMZ heterozygotes. Am. J. Respir. Crit. Care Med. 189, 419–427 (2014).
Sorheim, I. C. et al. α1-Antitrypsin protease inhibitor MZ heterozygosity is associated with airflow obstruction in two large cohorts. Chest 138, 1125–1132 (2010).
Piloni, D. et al. Comparison among populations with severe and intermediate alpha1-antitrypsin deficiency and chronic obstructive pulmonary disease. Minerva Med. 115, 23–31 (2024).
Raguram, A., Banskota, S. & Liu, D. R. Therapeutic in vivo delivery of gene editing agents. Cell 185, 2806–2827 (2022).
He, N. et al. Ferret models of alpha-1 antitrypsin deficiency develop lung and liver disease. JCI Insight 7, e143004 (2022).
Mandal, P. K. & Rossi, D. J. Reprogramming human fibroblasts to pluripotency using modified mRNA. Nat. Protoc. 8, 568–582 (2013).
Ryan, D. E. et al. Phosphonoacetate modifications enhance the stability and editing yields of guide RNAs for Cas9 editors. Biochemistry 62, 3512–3520 (2023).
Choi, J. et al. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell 27, 366–382 (2020).
Hasegawa, K. et al. Fraction of MHCII and EpCAM expression characterizes distal lung epithelial cells for alveolar type 2 cell isolation. Respir. Res. 18, 150 (2017).
Bae, S., Park, J. & Kim, J. S. Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 (2014).
Clement, K. et al. CRISPResso2 provides accurate and rapid genome editing sequence analysis. Nat. Biotechnol. 37, 224–226 (2019).
Muller, T. & Winter, D. Systematic evaluation of protein reduction and alkylation reveals massive unspecific side effects by iodine-containing reagents. Mol. Cell Proteomics. 16, 1173–1187 (2017).
Gundry, R. L. et al. Preparation of proteins and peptides for mass spectrometry analysis in a bottom-up proteomics workflow. Curr. Protoc. Mol. Biol. Chapter 10, Unit10.25 (2009).
Acknowledgements
This work was supported by a Sponsored Research Agreement with ReCode Therapeutics and the National Institutes of Health (NIH) National Institute of Biomedical Imaging and Bioengineering (NIBIB) (R01 EB025192-01A1). The UT Southwestern Small Animal Imaging Shared Resource is supported, in part, by the National Cancer Institute (P30CA142543) and the Cancer Prevention & Research Institute of Texas (RP210099). The authors would like to thank UT Southwestern Metabolic Phenotyping Core for the analysis of blood biochemicals; UT Southwestern Small Animal Imaging Resource for their shared AMI-HTX; UT Southwestern Tissue Management Shared Resource for helping with histology and H&E staining; UT Southwestern Proteomics Core for the analysis of A1AT isoforms; UT Southwestern Whole Brain Microscopy Facility for helping with slide scanning; the Moody Foundation Flow Cytometry Facility for helping with flow cytometry and providing expertise; and Inotiv histology and digital pathology services. The authors would also like to thank P. Hartono, L. Kahn, S. Sherman, M. Weinberg and J. Couch for their support.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.K. and D.J.S. Methodology: M.K. Investigation: M.K., E.S.S., J.C.C., S.C., Y.S., S.M.L., S.W., P.P., Z.T., A.K., B.A.W. and D.J.L. Visualization: M.K. and D.J.S. Resources: D.J.S. Project administration: D.J.S. Supervision: S.C. and D.J.S. Writing—original draft: M.K. and D.J.S. Writing—review and editing: D.J.S. Funding acquisition: D.J.S.
Corresponding author
Ethics declarations
Competing interests
A provisional patent application covering compositions, methods and uses for targeting cystic fibrosis and related disorders has been filed by UT Southwestern. D.J.S. is a co-founder and member of the scientific advisory board of ReCode Therapeutics, which has licensed intellectual property from UT Southwestern. D.J.S. discloses financial interests in ReCode Therapeutics, Signify Bio, Jumble Therapeutics and Tome Biosciences. J.C.C., A.K., B.A.W. and D.J.L. are employees of ReCode Therapeutics and have stock options in the company. All other authors declare that they have no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Keiichiro Suzuki and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Additional Supplementary Methods, DNA template sequences of Cas9 and ABEs, Methods-only references, Supplementary Figs. 1–17 and Supplementary Tables 1–5
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M., Song, E.S., Chen, J.C. et al. Dual SORT LNPs for multi-organ base editing. Nat Biotechnol (2025). https://doi.org/10.1038/s41587-025-02675-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-025-02675-z