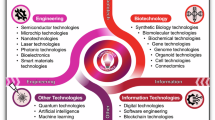
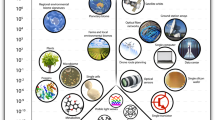
Abstract
Hybrid engineered biological systems integrate electronics and microfluidics with engineered biological components, such as microbes or cell-free DNA-based systems, to effectively sense, act upon and report on biological environments. As these engineered biological systems become essential in addressing challenges in healthcare, environmental monitoring, remediation and agriculture, this Review offers an in-depth discussion of their applications, critical design choices and challenges. Alongside an overview of the state of the field, we present a classification framework aimed at helping researchers make informed and optimized design decisions tailored to the intended biological application. Furthermore, we outline how the development of cyber-secure biological systems could enhance the security and functionality of engineered biological platforms. Last, we introduce a ‘Living Roadmap’ (https://www.programmingbiology.org/csbs) to dynamically reflect the field’s progress and support continuous monitoring of future advancements.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Cameron, D. E., Bashor, C. J. & Collins, J. J. A brief history of synthetic biology. Nat. Rev. Microbiol. 12, 381–390 (2014).
Brophy, J. A. N. & Voigt, C. A. Principles of genetic circuit design. Nat. Methods 11, 508–520 (2014).
Way, J. C., Collins, J. J., Keasling, J. D. & Silver, P. A. Integrating biological redesign: where synthetic biology came from and where it needs to go. Cell 157, 151–161 (2014).
Nielsen, J. & Keasling, J. D. Engineering cellular metabolism. Cell 164, 1185–1197 (2016).
Nielsen, A. A. K. et al. Genetic circuit design automation. Science 352, aac7341 (2016).
Jones, T. S., Oliveira, S. M. D., Myers, C. J., Voigt, C. A. & Densmore, D. Genetic circuit design automation with Cello 2.0. Nat. Protoc. 17, 1097–1113 (2022).
Angermueller, C., Pärnamaa, T., Parts, L. & Stegle, O. Deep learning for computational biology. Mol. Syst. Biol. 12, 878 (2016).
Shetty, R. P., Endy, D. & Knight, T. F. Jr. Engineering BioBrick vectors from BioBrick parts. J. Biol. Eng. 2, 5 (2008).
Purnick, P. E. M. & Weiss, R. The second wave of synthetic biology: from modules to systems. Nat. Rev. Mol. Cell Biol. 10, 410–422 (2009).
Kwok, R. Five hard truths for synthetic biology. Nature 463, 288–290 (2010).
Lee, S. Y. et al. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2, 18–33 (2019).
Keasling, J. D. Manufacturing molecules through metabolic engineering. Science 330, 1355–1358 (2010).
Khalil, A. S. & Collins, J. J. Synthetic biology: applications come of age. Nat. Rev. Genet. 11, 367–379 (2010).
Pei, L., Garfinkel, M. & Schmidt, M. Bottlenecks and opportunities for synthetic biology biosafety standards. Nat. Commun. 13, 2175 (2022).
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006).
Lan, F., Demaree, B., Ahmed, N. & Abate, A. R. Single-cell genome sequencing at ultra-high-throughput with microfluidic droplet barcoding. Nat. Biotechnol. 35, 640–646 (2017).
Abate, A. R., Hung, T., Mary, P., Agresti, J. J. & Weitz, D. A. High-throughput injection with microfluidics using picoinjectors. Proc. Natl Acad. Sci. USA 107, 19163–19166 (2010).
Selberg, J., Gomez, M. & Rolandi, M. The potential for convergence between synthetic biology and bioelectronics. Cell Syst. 7, 231–244 (2018).
Rivnay, J. et al. Integrating bioelectronics with cell-based synthetic biology. Nat. Rev. Bioeng. 3, 317–332 (2025).
Aghlmand, F. et al. A 65-nm CMOS fluorescence sensor for dynamic monitoring of living cells. IEEE J. Solid-State Circuits 58, 3003–3019 (2023).
Lee, H., Liu, Y., Westervelt, R. M. & Ham, D. IC/microfluidic hybrid system for magnetic manipulation of biological cells. IEEE J. Solid-State Circuits 41, 1471–1480 (2006).
Ghafar-Zadeh, E., Sawan, M., Chodavarapu, V. P. & Hosseini-Nia, T. Bacteria growth monitoring through a differential CMOS capacitive sensor. IEEE Trans. Biomed. Circuits Syst. 4, 232–238 (2010).
Manickam, A. et al. A fully integrated CMOS fluorescence biochip for DNA and RNA testing. IEEE J. Solid-State Circuits 52, 2857–2870 (2017).
Zhu, C., Maldonado, J. & Sengupta, K. CMOS-based electrokinetic microfluidics with multi-modal cellular and bio-molecular sensing for end-to-end point-of-care system. IEEE Trans. Biomed. Circuits Syst. 15, 1250–1267 (2021).
Inda-Webb, M. E. et al. Sub-1.4 cm3 capsule for detecting labile inflammatory biomarkers in situ. Nature 620, 386–392 (2023).
Tschirhart, T. et al. Electronic control of gene expression and cell behaviour in Escherichia coli through redox signalling. Nat. Commun. 8, 14030 (2017).
Eddie, B. J., Malanoski, A. P., Onderko, E. L., Phillips, D. A. & Glaven, S. M. Marinobacter atlanticus electrode biofilms differentially regulate gene expression depending on electrode potential and lifestyle. Biofilm 3, 100051 (2021).
Riglar, D. T. & Silver, P. A. Engineering bacteria for diagnostic and therapeutic applications. Nat. Rev. Microbiol. 16, 214–225 (2018).
Rothschild, L. J. et al. Building synthetic cells—from the technology infrastructure to cellular entities. ACS Synth. Biol. 13, 974–997 (2024).
Jung, J. K. et al. Cell-free biosensors for rapid detection of water contaminants. Nat. Biotechnol. 38, 1451–1459 (2020).
Takahashi, M. K. et al. A low-cost paper-based synthetic biology platform for analyzing gut microbiota and host biomarkers. Nat. Commun. 9, 3347 (2018).
Chen, Y. et al. Genetic circuit design automation for yeast. Nat. Microbiol. 5, 1349–1360 (2020).
Fedorec, A. J. H. et al. Emergent digital bio-computation through spatial diffusion and engineered bacteria. Nat. Commun. 15, 4896 (2024).
Sun, G. L., Reynolds, E. E. & Belcher, A. M. Designing yeast as plant-like hyperaccumulators for heavy metals. Nat. Commun. 10, 5080 (2019).
van der Meer, J. R. & Belkin, S. Where microbiology meets microengineering: design and applications of reporter bacteria. Nat. Rev. Microbiol. 8, 511–522 (2010).
Go´mez, R. et al. Microfluidic biochip for impedance spectroscopy of biological species. Biomed. Microdevices 3, 201–209 (2001).
Petchakup, C., Li, K. & Hou, H. Advances in single cell impedance cytometry for biomedical applications. Micromachines 8, 87 (2017).
Kang, J., Kim, T., Tak, Y., Lee, J.-H. & Yoon, J. Cyclic voltammetry for monitoring bacterial attachment and biofilm formation. J. Ind. Eng. Chem. 18, 800–807 (2012).
Zadeh, E. G., Sawan, M., Jalali, M. & Therriault, D. CMOS-based capacitive sensor array dedicated to microfluidic studies. In 2006 International Workshop on Computer Architecture for Machine Perception and Sensing 42–43 (IEEE, 2006).
Valijam, S. et al. Fabricating a dielectrophoretic microfluidic device using 3D-printed moulds and silver conductive paint. Sci. Rep. 13, 9560 (2023).
Akabuogu, E. U., Zhang, L., Krašovec, R., Roberts, I. S. & Waigh, T. A. Electrical impedance spectroscopy with bacterial biofilms: neuronal-like behavior. Nano Lett. 24, 2234–2241 (2024).
Arduini, F. et al. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 126, 346–354 (2019).
Yi, C., Li, C.-W., Ji, S. & Yang, M. Microfluidics technology for manipulation and analysis of biological cells. Anal. Chim. Acta 560, 1–23 (2006).
Gach, P. C. et al. A droplet microfluidic platform for automating genetic engineering. ACS Synth. Biol. 5, 426–433 (2016).
Iwai, K. et al. Scalable and automated CRISPR-based strain engineering using droplet microfluidics. Microsyst. Nanoeng. 8, 31 (2022).
Fu, A. Y., Spence, C., Scherer, A., Arnold, F. H. & Quake, S. R. A microfabricated fluorescence-activated cell sorter. Nat. Biotechnol. 17, 1109–1111 (1999).
Hatch, A. C. et al. 1-Million droplet array with wide-field fluorescence imaging for digital PCR. Lab Chip 11, 3838–3845 (2011).
Bhargava, K. C., Thompson, B. & Malmstadt, N. Discrete elements for 3D microfluidics. Proc. Natl Acad. Sci. USA 111, 15013–15018 (2014).
Zeng, Y., Novak, R., Shuga, J., Smith, M. T. & Mathies, R. A. High-performance single cell genetic analysis using microfluidic emulsion generator arrays. Anal. Chem. 82, 3183–3190 (2010).
Bennett, M. R. & Hasty, J. Microfluidic devices for measuring gene network dynamics in single cells. Nat. Rev. Genet. 10, 628–638 (2009).
Toriello, N. M. et al. Integrated microfluidic bioprocessor for single-cell gene expression analysis. Proc. Natl Acad. Sci. USA 105, 20173–20178 (2008).
Leung, K. et al. A programmable droplet-based microfluidic device applied to multiparameter analysis of single microbes and microbial communities. Proc. Natl Acad. Sci. USA 109, 7665–7670 (2012).
Churski, K. et al. Rapid screening of antibiotic toxicity in an automated microdroplet system. Lab Chip 12, 1629–1637 (2012).
Oblath, E. A., Henley, W. H., Alarie, J. P. & Ramsey, J. M. A microfluidic chip integrating DNA extraction and real-time PCR for the detection of bacteria in saliva. Lab Chip 13, 1325–1332 (2013).
Lashkaripour, A. et al. Machine learning enables design automation of microfluidic flow-focusing droplet generation. Nat. Commun. 12, 25 (2021).
Linshiz, G. et al. End-to-end automated microfluidic platform for synthetic biology: from design to functional analysis. J. Biol. Eng. 10, 3 (2016).
Fracassi, C., Postiglione, L., Fiore, G. & di Bernardo, D. Automatic control of gene expression in mammalian cells. ACS Synth. Biol. 5, 296–302 (2015).
Wu, L. L., Babikian, S., Li, G.-P. & Bachman, M. Microfluidic printed circuit boards. In 2011 IEEE 61st Electronic Components and Technology Conference (ECTC) 1576–1581 (IEEE, 2011).
Husser, M. C., Vo, P. Q. N., Sinha, H., Ahmadi, F. & Shih, S. C. C. An automated induction microfluidics system for synthetic biology. ACS Synth. Biol. 7, 933–944 (2018).
Howell, J., Hammarton, T. C., Altmann, Y. & Jimenez, M. High-speed particle detection and tracking in microfluidic devices using event-based sensing. Lab Chip 20, 3024–3035 (2020).
de Cesare, I. et al. ChipSeg: an automatic tool to segment bacterial and mammalian cells cultured in microfluidic devices. ACS Omega 6, 2473–2476 (2021).
Bachler, S., Haidas, D., Ort, M., Duncombe, T. A. & Dittrich, P. S. Microfluidic platform enables tailored translocation and reaction cascades in nanoliter droplet networks. Commun. Biol. 3, 769 (2020).
van Sluijs, B., Maas, R. J. M., van der Linden, A. J., de Greef, T. F. A. & Huck, W. T. S. A microfluidic optimal experimental design platform for forward design of cell-free genetic networks. Nat. Commun. 13, 3626 (2022).
Zhao, S. et al. A new design for living cell-based biosensors: microgels with a selectively permeable shell that can harbor bacterial species. Sens. Actuators B Chem. 334, 129648 (2021).
Khazim, M., Pedone, E., Postiglione, L., di Bernardo, D. & Marucci, L. A microfluidic/microscopy-based platform for on-chip controlled gene expression in mammalian cells. Methods Mol. Biol. 2229, 205–219 (2021).
Ren, Y. et al. A three-in-one microfluidic droplet digital PCR platform for absolute quantitative analysis of DNA. Lab Chip 23, 2521–2530 (2023).
Sun, Y. et al. Two-layered microfluidic devices for high-throughput dynamic analysis of synthetic gene circuits in E. coli. ACS Synth. Biol. 11, 3954–3965 (2022).
Rahman, K. M. T. & Butzin, N. C. Counter-on-chip for bacterial cell quantification, growth, and live–dead estimations. Sci. Rep. 14, 782 (2024).
Pardee, K. et al. Paper-based synthetic gene networks. Cell 159, 940–954 (2014).
Dou, M., Dominguez, D. C., Li, X., Sanchez, J. & Scott, G. A versatile PDMS/paper hybrid microfluidic platform for sensitive infectious disease diagnosis. Anal. Chem. 86, 7978–7986 (2014).
Delamarche, E., Juncker, D. & Schmid, H. Microfluidics for processing surfaces and miniaturizing biological assays. Adv. Mater. 17, 2911–2933 (2005).
Gulati, S. et al. Opportunities for microfluidic technologies in synthetic biology. J. R. Soc. Interface 6, S493–S506 (2009).
Xian, Z. et al. A novel microfluidics PMMA/paper hybrid bioimmunosensor for laser-induced fluorescence detection in the determination of α-fetoprotein from serum. Microchem. J. 195, 109476 (2023).
Srinivasan, V., Pamula, V., Pollack, M. & Fair, R. A digital microfluidic biosensor for multianalyte detection. In the Sixteenth Annual International Conference on Micro Electro Mechanical Systems 327–330 (IEEE, 2003).
Hamedi, M. M. et al. Integrating electronics and microfluidics on paper. Adv. Mater. 28, 5054–5063 (2016).
Lee, H., Sun, E., Ham, D. & Weissleder, R. Chip–NMR biosensor for detection and molecular analysis of cells. Nat. Med. 14, 869–874 (2008).
Zhou, A. Y., Baruch, M., Ajo-Franklin, C. M. & Maharbiz, M. M. A portable bioelectronic sensing system (BESSY) for environmental deployment incorporating differential microbial sensing in miniaturized reactors. PLoS ONE 12, e0184994 (2017).
Madhvapathy, S. R. et al. Miniaturized implantable temperature sensors for the long-term monitoring of chronic intestinal inflammation. Nat. Biomed. Eng. 8, 1040–1052 (2024).
Stephenson, A. et al. PurpleDrop: a digital microfluidics-based platform for hybrid molecular–electronics applications. IEEE Micro 40, 76–86 (2020).
Cai, R. et al. Creation of a point-of-care therapeutics sensor using protein engineering, electrochemical sensing and electronic integration. Nat. Commun. 15, 1689 (2024).
Coelho, B. et al. Hybrid digital-droplet microfluidic chip for applications in droplet digital nucleic acid amplification: design, fabrication and characterization. Sensors 23, 4927 (2023).
Bouzid, K., Greener, J., Carrara, S. & Gosselin, B. Portable impedance-sensing device for microorganism characterization in the field. Sci. Rep. 13, 10526 (2023).
Jafari, H., Soleymani, L. & Genov, R. 16-Channel CMOS impedance spectroscopy DNA analyzer with dual-slope multiplying ADCs. IEEE Trans. Biomed. Circuits Syst. 6, 468–478 (2012).
Manaresi, N. et al. A CMOS chip for individual cell manipulation and detection. IEEE J. Solid-State Circuits 38, 2297–2305 (2003).
Luan, L., Evans, R. D., Jokerst, N. M. & Fair, R. B. Integrated optical sensor in a digital microfluidic platform. IEEE Sens. J. 8, 628–635 (2008).
Hunt, T. P., Issadore, D. & Westervelt, R. M. Integrated circuit/microfluidic chip to programmably trap and move cells and droplets with dielectrophoresis. Lab Chip 8, 81–87 (2008).
Lai, K. Y.-T., Yang, Y.-T. & Lee, C.-Y. An intelligent digital microfluidic processor for biomedical detection. J. Signal Process. Syst. 78, 85–93 (2014).
Park, J. et al. Microscale biosensor array based on flexible polymeric platform toward lab-on-a-needle: real-time multiparameter biomedical assays on curved needle surfaces. ACS Sens. 5, 1363–1373 (2020).
Khorasani, M., Behnam, M., van den Berg, L., Backhouse, C. J. & Elliott, D. G. High-voltage CMOS controller for microfluidics. IEEE Trans. Biomed. Circuits Syst. 3, 89–96 (2009).
Issadore, D., Franke, T., Brown, K. A. & Westervelt, R. M. A microfluidic microprocessor: controlling biomimetic containers and cells using hybrid integrated circuit/microfluidic chips. Lab Chip 10, 2937–2943 (2010).
Manickam, A., Chevalier, A., McDermott, M., Ellington, A. D. & Hassibi, A. A CMOS electrochemical impedance spectroscopy (EIS) biosensor array. IEEE Trans. Biomed. Circuits Syst. 4, 379–390 (2010).
Bounik, R. et al. A CMOS microelectrode array integrated into an open, continuously perfused microfluidic system. In 2022 IEEE Biomedical Circuits and Systems Conference (BioCAS) 491–494 (IEEE, 2022).
Ding, Z., Xu, C., Wang, Y. & Pellegrini, G. Ultra-low-light CMOS biosensor complements microfluidics to achieve portable diagnostics. Procedia Technol. 27, 39–41 (2017).
Frey, U. et al. Switch-matrix-based high-density microelectrode array in CMOS technology. IEEE J. Solid-State Circuits 45, 467–482 (2010).
Liu, Q. et al. 17.7 Droplet microfluidics co-designed with real-time CMOS luminescence sensing and impedance spectroscopy of 4nl droplets at a 67mm/s velocity. In 2024 IEEE International Solid-State Circuits Conference (ISSCC) 326–328 (IEEE, 2024).
Jin, X., Liu, Z., Li, T., Guo, Q. & Yang, J. Online monitoring and portable analytical system with CMOS sensor and microfluidic technology for cell cultivation applications. In 2010 Symposium on Photonics and Optoelectronics 1–4 (IEEE, 2010).
Issadore, D., Franke, T., Brown, K. A., Hunt, T. P. & Westervelt, R. M. High-voltage dielectrophoretic and magnetophoretic hybrid integrated circuit/microfluidic chip. J. Microelectromech. Syst. 18, 1220–1225 (2009).
Li, R. et al. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 11, 3207 (2020).
Uguz, I. et al. Flexible switch matrix addressable electrode arrays with organic electrochemical transistor and pn diode technology. Nat. Commun. 15, 533 (2024).
Sawan, M., Miled, M. A. & Ghafar-Zadeh, E. CMOS/microfluidic lab-on-chip for cells-based diagnostic tools. In 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology 5334–5337 (IEEE, 2010).
Cornelis, S. et al. Silicon µPCR chip for forensic STR profiling with hybeacon probe melting curves. Sci. Rep. 9, 7341 (2019).
Mimee, M. et al. An ingestible bacterial–electronic system to monitor gastrointestinal health. Science 360, 915–918 (2018).
Lee, H., Liu, Y., Ham, D. & Westervelt, R. M. Integrated cell manipulation system—CMOS/microfluidic hybrid. Lab Chip 7, 331–337 (2007).
Zhou, Q. et al. Miniature magnetic resonance imaging system for in situ monitoring of bacterial growth and biofilm formation. In IEEE Transactions on Biomedical Circuits and Systems 990–1000 (IEEE, 2024).
Hall, D. A. et al. A scalable CMOS molecular electronics chip for single-molecule biosensing. IEEE Trans. Biomed. Circuits Syst. 16, 1030–1043 (2022).
Huang, Y. & Mason, A. J. Lab-on-CMOS integration of microfluidics and electrochemical sensors. Lab Chip 13, 3929–3934 (2013).
Ghafar-Zadeh, E., Sawan, M. & Therriault, D. Novel direct-write CMOS-based laboratory-on-chip: design, assembly and experimental results. Sens. Actuators A Phys. 134, 27–36 (2007).
Lee, H., Xu, L., Koh, D., Nyayapathi, N. & Oh, K. Various on-chip sensors with microfluidics for biological applications. Sensors 14, 17008–17036 (2014).
Toumazou, C. et al. Simultaneous DNA amplification and detection using a pH-sensing semiconductor system. Nat. Methods 10, 641–646 (2013).
Chien, J.-C. et al. A high-throughput flow cytometry-on-a-CMOS platform for single-cell dielectric spectroscopy at microwave frequencies. Lab Chip 18, 2065–2076 (2018).
Levine, P. M., Gong, P., Levicky, R. & Shepard, K. L. Active CMOS sensor array for electrochemical biomolecular detection. IEEE J. Solid-State Circuits 43, 1859–1871 (2008).
Rosenstein, J. K., Wanunu, M., Merchant, C. A., Drndic, M. & Shepard, K. L. Integrated nanopore sensing platform with sub-microsecond temporal resolution. Nat. Methods 9, 487–492 (2012).
Hong, L., Li, H., Yang, H. & Sengupta, K. Fully integrated fluorescence biosensors on-chip employing multi-functional nanoplasmonic optical structures in CMOS. IEEE J. Solid-State Circuits 52, 2388–2406 (2017).
Zhu, C., Wen, Y., Liu, T., Yang, H. & Sengupta, K. An ingestible pill with CMOS fluorescence sensor array, bi-directional wireless interface and packaged optics for in-vivo bio-molecular sensing. IEEE Trans. Biomed. Circuits Syst. 17, 257–272 (2023).
Bustillo, J., Fife, K., Merriman, B. & Rothberg, J. Development of the ion torrent CMOS chip for DNA sequencing. In 2013 IEEE International Electron Devices Meeting 8.1.1–8.1.4 (IEEE, 2013).
Lai, K. Y.-T. et al. A field-programmable lab-on-a-chip with built-in self-test circuit and low-power sensor-fusion solution in 0.35μm standard CMOS process. In 2015 IEEE Asian Solid-State Circuits Conference (A-SSCC) 1–4 (IEEE, 2015).
Murali, P. et al. 24.6 A CMOS micro-flow cytometer for magnetic label detection and classification. In 2014 IEEE International Solid-State Circuits Conference Digest of Technical Papers (ISSCC) 422–423 (IEEE, 2014).
Singh, R. R., Leng, L., Guenther, A. & Genov, R. A CMOS–microfluidic chemiluminescence contact imaging microsystem. IEEE J. Solid-State Circuits 47, 2822–2833 (2012).
Kumashi, S. et al. A CMOS multi-modal electrochemical and impedance cellular sensing array for massively paralleled exoelectrogen screening. IEEE Trans. Biomed. Circuits Syst. 15, 221–234 (2021).
Lee, D. et al. A multi-functional CMOS biosensor array with on-chip DEP-assisted sensing for rapid low-concentration analyte detection and close-loop particle manipulation with no external electrodes. IEEE Trans. Biomed. Circuits Syst. 17, 1214–1226 (2023).
Kuo, Y.-H., Chen, Y.-S., Huang, P.-C. & Lee, G.-B. A CMOS-based capacitive biosensor for detection of a breast cancer microRNA biomarker. IEEE Open J. Nanotechnol. 1, 157–162 (2020).
Murari, K., Etienne-Cummings, R., Thakor, N. V. & Cauwenberghs, G. A CMOS in-pixel CTIA high-sensitivity fluorescence imager. IEEE Trans. Biomed. Circuits Syst. 5, 449–458 (2011).
Forouhi, S., Dehghani, R. & Ghafar-Zadeh, E. CMOS based capacitive sensors for life science applications: a review. Sens. Actuators A Phys. 297, 111531 (2019).
Vallero, A. et al. Memristive biosensors integration with microfluidic platform. IEEE Trans. Circuits Syst. I Regul. Pap. 63, 2120–2127 (2016).
Sun, A. C., Alvarez-Fontecilla, E., Venkatesh, A. G., Aronoff-Spencer, E. & Hall, D. A. High-density redox amplified coulostatic discharge-based biosensor array. IEEE J. Solid-State Circuits 53, 2054–2064 (2018).
Tang, H. et al. 2D magnetic sensor array for real-time cell tracking and multi-site detection with increased robustness and flow-rate. In 2019 IEEE Custom Integrated Circuits Conference (CICC) 1–4 (IEEE, 2019).
Spyropoulos, G. D., Gelinas, J. N. & Khodagholy, D. Internal ion-gated organic electrochemical transistor: a building block for integrated bioelectronics. Sci. Adv. 5, eaau7378 (2019).
Linder, V. et al. Microfluidics/CMOS orthogonal capabilities for cell biology. Biomed. Microdevices 8, 159–166 (2006).
Atkinson, J. T. et al. Real-time bioelectronic sensing of environmental contaminants. Nature 611, 548–553 (2022).
Hall, D. A., Gaster, R. S., Makinwa, K. A. A., Wang, S. X. & Murmann, B. A 256 pixel magnetoresistive biosensor microarray in 0.18 µm CMOS. IEEE J. Solid-State Circuits 48, 1290–1301 (2013).
Fuller, C. W. et al. Molecular electronics sensors on a scalable semiconductor chip: a platform for single-molecule measurement of binding kinetics and enzyme activity. Proc. Natl Acad. Sci. USA 119, e2112812119 (2022).
Manickam, A. et al. A fully-electronic charge-based DNA sequencing CMOS biochip. In 2012 Symposium on VLSI Circuits (VLSIC) 126–127 (IEEE, 2012).
Manickam, A. et al. A CMOS electrochemical biochip with 32 × 32 three-electrode voltammetry pixels. IEEE J. Solid-State Circuits 54, 2980–2990 (2019).
Dragas, J. et al. In vitro multi-functional microelectrode array featuring 59760 electrodes, 2048 electrophysiology channels, stimulation, impedance measurement, and neurotransmitter detection channels. IEEE J. Solid-State Circuits 52, 1576–1590 (2017).
Rothe, J., Frey, O., Stettler, A., Chen, Y. & Hierlemann, A. Fully integrated CMOS microsystem for electrochemical measurements on 32 × 32 working electrodes at 90 frames per second. Anal. Chem. 86, 6425–6432 (2014).
Wang, H., Mahdavi, A., Tirrell, D. A. & Hajimiri, A. A magnetic cell-based sensor. Lab Chip 12, 4465–4471 (2012).
Lee, D. et al. 17.6 Fully integrated CMOS ferrofluidic biomolecular processing platform with on-chip droplet-based manipulation, multiplexing and sensing. In 2024 IEEE International Solid-State Circuits Conference (ISSCC) 324–326 (IEEE, 2024).
Hierlemann, A., Frey, U., Hafizovic, S. & Heer, F. Growing cells atop microelectronic chips: interfacing electrogenic cells in vitro with CMOS-based microelectrode arrays. Proc. IEEE 99, 252–284 (2011).
Welch, D. & Christen, J. B. Seamless integration of CMOS and microfluidics using flip chip bonding. J. Micromech. Microeng. 23, 035009 (2013).
Dong, R., Liu, Y., Mou, L., Deng, J. & Jiang, X. Microfluidics‐based biomaterials and biodevices. Adv. Mater. 31, e1805033 (2018).
van Erp, R., Soleimanzadeh, R., Nela, L., Kampitsis, G. & Matioli, E. Co-designing electronics with microfluidics for more sustainable cooling. Nature 585, 211–216 (2020).
Sun, T. & Morgan, H. Single-cell microfluidic impedance cytometry: a review. Microfluid. Nanofluid. 8, 423–443 (2010).
Ostrov, N. et al. A modular yeast biosensor for low-cost point-of-care pathogen detection. Sci. Adv. 3, e1603221 (2017).
Carpenter, A. C., Paulsen, I. T. & Williams, T. C. Blueprints for biosensors: design, limitations, and applications. Genes 9, 375 (2018).
Au, A. K., Bhattacharjee, N., Horowitz, L. F., Chang, T. C. & Folch, A. 3D-printed microfluidic automation. Lab Chip 15, 1934–1941 (2015).
Paguirigan, A. L. & Beebe, D. J. Microfluidics meet cell biology: bridging the gap by validation and application of microscale techniques for cell biological assays. BioEssays 30, 811–821 (2008).
Yan, S., Zhang, J., Yuan, D. & Li, W. Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 38, 238–249 (2016).
Battat, S., Weitz, D. A. & Whitesides, G. M. An outlook on microfluidics: the promise and the challenge. Lab Chip 22, 530–536 (2022).
Atkinson, J. T., Chavez, M. S., Niman, C. M. & El‐Naggar, M. Y. Living electronics: a catalogue of engineered living electronic components. Microb. Biotechnol. 16, 507–533 (2022).
Diorio, C., Hsu, D. & Figueroa, M. Adaptive CMOS: from biological inspiration to systems-on-a-chip. Proc. IEEE 90, 345–357 (2002).
Mosadegh, B., Bersano-Begey, T., Park, J. Y., Burns, M. A. & Takayama, S. Next-generation integrated microfluidic circuits. Lab Chip 11, 2813–2818 (2011).
Lashkaripour, A., Silva, R. & Densmore, D. Desktop micromilled microfluidics. Microfluid. Nanofluid. 22, 31 (2018).
Khan, S. M., Gumus, A., Nassar, J. M. & Hussain, M. M. CMOS enabled microfluidic systems for healthcare based applications. Adv. Mater. 30, e1705759 (2018).
Karim, A. S. et al. Deconstructing synthetic biology across scales: a conceptual approach for training synthetic biologists. Nat. Commun. 15, 5425 (2024).
Ma, Y. et al. A review of electrochemical electrodes and readout interface designs for biosensors. IEEE Open J. Solid-State Circuits Soc. 3, 76–88 (2023).
Dixon, T. A., Williams, T. C. & Pretorius, I. S. Sensing the future of bio-informational engineering. Nat. Commun. 12, 388 (2021).
Datta-Chaudhuri, T., Smela, E. & Abshire, P. A. System-on-chip considerations for heterogeneous integration of CMOS and fluidic bio-interfaces. IEEE Trans. Biomed. Circuits Syst. 10, 1129–1142 (2016).
Brooks, S. M. & Alper, H. S. Applications, challenges, and needs for employing synthetic biology beyond the lab. Nat. Commun. 12, 1390 (2021).
Steiger, C. et al. Ingestible electronics for diagnostics and therapy. Nat. Rev. Mater. 4, 83–98 (2018).
Nadeau, P. et al. Prolonged energy harvesting for ingestible devices. Nat. Biomed. Eng. 1, 0022 (2017).
Muluneh, M. & Issadore, D. A multi-scale PDMS fabrication strategy to bridge the size mismatch between integrated circuits and microfluidics. Lab Chip 14, 4552–4558 (2014).
Zargaryan, A., Farhoudi, N., Haworth, G., Ashby, J. F. & Au, S. H. Hybrid 3D printed-paper microfluidics. Sci. Rep. 10, 18379 (2020).
Huang, H. & Densmore, D. Fluigi. ACM J. Emerg. Technol. Comput. Syst. 11, 1–19 (2014).
Kim, S. J. et al. The bottom of the memory hierarchy: semiconductor and DNA data storage. MRS Bull. 48, 547–559 (2023).
Ros, P. M., Miccoli, B., Sanginario, A. & Demarchi, D. Low-power architecture for integrated CMOS bio-sensing. In 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS) 1–4 (IEEE, 2017).
Perry, J. M., Soffer, G., Jain, R. & Shih, S. C. C. Expanding the limits towards ‘one-pot’ DNA assembly and transformation on a rapid-prototype microfluidic device. Lab Chip 21, 3730–3741 (2021).
Ahrar, S., Raje, M., Lee, I. C. & Hui, E. E. Pneumatic computers for embedded control of microfluidics. Sci. Adv. 9, eadg0201 (2023).
Iyer, V., Murali, P., Paredes, J., Liepmann, D. & Boser, B. Encapsulation of integrated circuits in plastic microfluidic systems using hot embossing. In 2015 Transducers — 2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS) 1822–1825 (IEEE, 2015).
Gopinathan, K. A., Mishra, A., Mutlu, B. R., Edd, J. F. & Toner, M. A microfluidic transistor for automatic control of liquids. Nature 622, 735–741 (2023).
Jang, B. & Hassibi, A. Biosensor systems in standard CMOS processes: fact or fiction? In 2008 IEEE International Symposium on Industrial Electronics 2045–2050 (IEEE, 2008).
Singh, R., Manickam, A. & Hassibi, A. CMOS biochips for hypothesis-driven DNA analysis. In 2014 IEEE Biomedical Circuits and Systems Conference (BioCAS) Proceedings 484–487 (IEEE, 2014).
Olanrewaju, A., Beaugrand, M., Yafia, M. & Juncker, D. Capillary microfluidics in microchannels: from microfluidic networks to capillaric circuits. Lab Chip 18, 2323–2347 (2018).
Li, J., Ha, N. S., ‘Leo’ Liu, T., van Dam, R. M. & Kim, C.-J. ‘C. J. ’ Ionic-surfactant-mediated electro-dewetting for digital microfluidics. Nature 572, 507–510 (2019).
Pollack, M. G., Shenderov, A. D. & Fair, R. B. Electrowetting-based actuation of droplets for integrated microfluidics. Lab Chip 2, 96 (2002).
Moragues, T. et al. Droplet-based microfluidics. Nat. Rev. Methods Primers 3, 32 (2023).
Ding, Y., Howes, P. D. & deMello, A. J. Recent advances in droplet microfluidics. Anal. Chem. 92, 132–149 (2019).
Lenshof, A. & Laurell, T. Continuous separation of cells and particles in microfluidic systems. Chem. Soc. Rev. 39, 1203–1217 (2010).
Beebe, D. J., Mensing, G. A. & Walker, G. M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 4, 261–286 (2002).
Nielsen, J. B. et al. Microfluidics: innovations in materials and their fabrication and functionalization. Anal. Chem. 92, 150–168 (2019).
Tsur, E. E. Computer-aided design of microfluidic circuits. Annu. Rev. Biomed. Eng. 22, 285–307 (2020).
Sanka, R., Lippai, J., Samarasekera, D., Nemsick, S. & Densmore, D. 3DμF — interactive design environment for continuous flow microfluidic devices. Sci. Rep. 9, 9166 (2019).
Squires, T. M. & Quake, S. R. Microfluidics: fluid physics at the nanoliter scale. Rev. Mod. Phys. 77, 977–1026 (2005).
Sackmann, E. K., Fulton, A. L. & Beebe, D. J. The present and future role of microfluidics in biomedical research. Nature 507, 181–189 (2014).
Liu, Q. et al. A threshold-based bioluminescence detector with a CMOS-integrated photodiode array in 65 nm for a multi-diagnostic ingestible capsule. IEEE J. Solid-State Circuits 58, 838–851 (2023).
Gregor, C., Gwosch, K. C., Sahl, S. J. & Hell, S. W. Strongly enhanced bacterial bioluminescence with the ilux operon for single-cell imaging. Proc. Natl Acad. Sci. USA 115, 962–967 (2018).
Ying, D. & Hall, D. A. Current sensing front-ends: a review and design guidance. IEEE Sens. J. 21, 22329–22346 (2021).
Mulleti, S., Bhandari, A. & Eldar, Y. C. Power-aware analog to digital converters. In Applied and Numerical Harmonic Analysis 415–452 (Birkhäuser, 2023).
Yasar, A. & Yazicigil, R. T. Physical-layer security for energy-constrained integrated systems: challenges and design perspectives. IEEE Open J. Solid-State Circuits Soc. 3, 262–273 (2023).
De la Paz, E. et al. A self-powered ingestible wireless biosensing system for real-time in situ monitoring of gastrointestinal tract metabolites. Nat. Commun. 13, 7405 (2022).
Chandrakasan, A. P., Verma, N. & Daly, D. C. Ultralow-power electronics for biomedical applications. Annu. Rev. Biomed. Eng. 10, 247–274 (2008).
Mercier, P. P., Lysaght, A. C., Bandyopadhyay, S., Chandrakasan, A. P. & Stankovic, K. M. Energy extraction from the biologic battery in the inner ear. Nat. Biotechnol. 30, 1240–1243 (2012).
Wang, A., Highsmith Calhoun, B., & Chandrakasan, A. P. Sub-threshold design for ultra low-power systems. In Series on Integrated Circuits and Systems (Springer, 2006).
Farrar, J. T., Berkley, C. & Zworykin, V. K. Telemetering of intraenteric pressure in man by an externally energized wireless capsule. Science 131, 1814 (1960).
Yeknami, A. F. et al. A 0.3-V CMOS biofuel-cell-powered wireless glucose/lactate biosensing system. IEEE J. Solid-State Circuits 53, 3126–3139 (2018).
Dong, K. et al. Microbial fuel cell as power supply for implantable medical devices: a novel configuration design for simulating colonic environment. Biosens. Bioelectron. 41, 916–919 (2013).
El-Damak, D. & Chandrakasan, A. P. Solar energy harvesting system with integrated battery management and startup using single inductor and 3.2nW quiescent power. In 2015 Symposium on VLSI Circuits (VLSI Circuits) C280–C281 (IEEE, 2015).
Kadirvel, K. et al. A 330nA energy-harvesting charger with battery management for solar and thermoelectric energy harvesting. In 2012 IEEE International Solid-State Circuits Conference - (ISSCC) 106–108 (IEEE, 2012).
Ramadass, Y. K. & Chandrakasan, A. P. A batteryless thermoelectric energy-harvesting interface circuit with 35mV startup voltage. In 2010 IEEE International Solid-State Circuits Conference — (ISSCC) 486–487 (IEEE, 2010).
Dagdeviren, C. et al. Conformal piezoelectric energy harvesting and storage from motions of the heart, lung, and diaphragm. Proc. Natl Acad. Sci. USA 111, 1927–1932 (2014).
Sadat Mousavi, P. et al. A multiplexed, electrochemical interface for gene-circuit-based sensors. Nat. Chem. 12, 48–55 (2019).
Amalfitano, E. et al. A glucose meter interface for point-of-care gene circuit-based diagnostics. Nat. Commun. 12, 724 (2021).
Sang, M., Kim, K., Shin, J. & Yu, K. J. Ultra‐thin flexible encapsulating materials for soft bio‐integrated electronics. Adv. Sci. 9, e2202980 (2022).
Yang, Y. & Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491 (2019).
Iyer, V., Issadore, D. A. & Aflatouni, F. The next generation of hybrid microfluidic/integrated circuit chips: recent and upcoming advances in high-speed, high-throughput, and multifunctional lab-on-IC systems. Lab Chip 23, 2553–2576 (2023).
McClune, C. J., Alvarez-Buylla, A., Voigt, C. A. & Laub, M. T. Engineering orthogonal signalling pathways reveals the sparse occupancy of sequence space. Nature 574, 702–706 (2019).
Du, P. et al. De novo design of an intercellular signaling toolbox for multi-channel cell–cell communication and biological computation. Nat. Commun. 11, 4226 (2020).
Marken, J. P. & Murray, R. M. Addressable and adaptable intercellular communication via DNA messaging. Nat. Commun. 14, 2358 (2023).
Sexton, J. T. & Tabor, J. J. Multiplexing cell–cell communication. Mol. Syst. Biol. 16, e9618 (2020).
LaFleur, T. L., Hossain, A. & Salis, H. M. Automated model-predictive design of synthetic promoters to control transcriptional profiles in bacteria. Nat. Commun. 13, 5159 (2022).
Sun, M. G. F., Seo, M.-H., Nim, S., Corbi-Verge, C. & Kim, P. M. Protein engineering by highly parallel screening of computationally designed variants. Sci. Adv. 2, e1600692 (2016).
Roehner, N. et al. GOLDBAR: a framework for combinatorial biological design. ACS Synth. Biol. 13, 2899–2911 (2024).
Naseri, G. & Koffas, M. A. G. Application of combinatorial optimization strategies in synthetic biology. Nat. Commun. 11, 2446 (2020).
Salahuddin, S., Ni, K. & Datta, S. The era of hyper-scaling in electronics. Nat. Electron. 1, 442–450 (2018).
Castle, S. D., Stock, M. & Gorochowski, T. E. Engineering is evolution: a perspective on design processes to engineer biology. Nat. Commun. 15, 3640 (2024).
Oliveira, S. M. D. & Densmore, D. Hardware, software, and wetware codesign environment for synthetic biology. Biodes. Res. 2022, 9794510 (2022).
Yazicigil, R. T. et al. Beyond crypto: physical-layer security for internet of things devices. IEEE Solid-State Circuits Mag. 12, 66–78 (2020).
Vakhter, V., Soysal, B., Schaumont, P. & Guler, U. Threat modeling and risk analysis for miniaturized wireless biomedical devices. IEEE Internet Things J. 9, 13338–13352 (2022).
Vatambeti, R. et al. Prediction of DDoS attacks in Agriculture 4.0 with the help of prairie dog optimization algorithm with IDSNet. Sci. Rep. 13, 15371 (2023).
Maraveas, C., Rajarajan, M., Arvanitis, K. G. & Vatsanidou, A. Cybersecurity threats and mitigation measures in Agriculture 4.0 and 5.0. Smart Agric. Technol. 9, 100616 (2024).
Rettore de Araujo Zanella, A., da Silva, E. & Pessoa Albini, L. C. Security challenges to smart agriculture: current state, key issues, and future directions. Array 8, 100048 (2020).
Acknowledgements
We thank D. Arguijo, Q. Liu, J. Yepes, J. Chen, D. Vance, H. Gordon, E. Yimer Wolle, A. Yasar, A. Pemaraj, Y. Patel, A. Skerker, Y. Zhou, N. Markowitz, A. Kassem, J. Roberts, Z. Kizilates and M. Jimenez, E. Zheng and H. Xiong. Sponsored by the National Science Foundation SemiSynBio-II Program (to D.D. and R.T.Y., award 2027045), the National Science Foundation CAREER Program (to R.T.Y. and D.C., award 2338792), the Semiconductor Research Corporation ESH Program (to D.D., R.T.Y. and D.C., task 3245.001) and the Catalyst Foundation (to D.D., R.T.Y. and D.C., SAP grant 55208844). We thank the Boston University Biological Design Center for supporting the engineered biology research infrastructure.
Author information
Authors and Affiliations
Contributions
R.T.Y. and D.D. wrote the manuscript; A.B., D.D. and R.T.Y. contributed to data analysis; D.C., D.D. and R.T.Y. contributed to data visualization; A.B., D.D. and R.T.Y. contributed to ‘Living Roadmap’ website design and development.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks Yuanjin Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Website and Figs. 1 and 2
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yazicigil, R.T., Bali, A., Caygara, D. et al. Improving engineered biological systems with electronics and microfluidics. Nat Biotechnol (2025). https://doi.org/10.1038/s41587-025-02709-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41587-025-02709-6