Abstract
αβ T cell antigen receptors (TCRs) bind complexes of peptide and major histocompatibility complex (pMHC) with low affinity, which poses a considerable challenge for the direct identification of αβ T cell cognate peptides. Here we describe a platform for the discovery of MHC class II epitopes based on the screening of engineered reporter cells expressing novel pMHC–TCR (MCR) hybrid molecules carrying cDNA-derived peptides. This technology identifies natural epitopes of CD4+ T cells in an unbiased and efficient manner and allows detailed analysis of TCR cross-reactivity that provides recognition patterns beyond discrete peptides. We determine the cognate peptides of virus- and tumor-specific T cells in mouse disease models and present a proof of concept for human T cells. Furthermore, we use MCR to identify immunogenic tumor neo-antigens and show that vaccination with a peptide naturally recognized by tumor-infiltrating lymphocytes efficiently protects mice from tumor challenge. Thus, the MCR technology holds promise for basic research and clinical applications, allowing the personalized identification of T cell–specific neo-antigens in patients.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Sequence data generated in this study were deposited as Fastq files at the European Nucleotide Archive (https://www.ebi.ac.uk/ena) under accession codes ERS3019426, ERS3019427, ERS3019428 and ERS3019429 (B6 R1, B6 R2, E0771 R1 and E0771 R2, respectively) and study accession code PRJEB30681.
Change history
03 April 2019
In the version of this article initially published, a reference (23) was cited incorrectly and two references were not included in the second sentence of the first paragraph of the second Results subsection (‘Screening for gp61 mimotopes with different functional properties’). The correct citation is as follows: “... we replaced the very stable GFP with a slow fluorescent timer (FT)27,28.” Full details on the added references can be found in the correction notice. The errors have been corrected in the print, PDF and HTML versions of the paper.
References
Linnemann, C. et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 21, 81–85 (2015).
Gundlach, B. R. et al. Determination of T cell epitopes with random peptide libraries. J. Immunol. Methods 192, 149–155 (1996).
Borras, E. et al. Findings on T cell specificity revealed by synthetic combinatorial libraries. J. Immunol. Methods 267, 79–97 (2002).
Dogan, I. et al. Phage-displayed libraries of peptide/major histocompatibility complexes. Eur. J. Immunol. 34, 598–607 (2004).
Crawford, F., Huseby, E., White, J., Marrack, P. & Kappler, J. W. Mimotopes for alloreactive and conventional T cells in a peptide–MHC display library. PLoS Biol. 2, e90 (2004).
Wen, F., Esteban, O. & Zhao, H. Rapid identification of CD4+ T-cell epitopes using yeast displaying pathogen-derived peptide library. J. Immunol. Methods 336, 37–44 (2008).
Siewert, K. et al. Unbiased identification of target antigens of CD8+ T cells with combinatorial libraries coding for short peptides. Nat. Med. 18, 824–828 (2012).
Newell, E. W. et al. Combinatorial tetramer staining and mass cytometry analysis facilitate T-cell epitope mapping and characterization. Nat. Biotechnol. 31, 623–629 (2013).
Birnbaum, M. E., Dong, S. & Garcia, K. C. Diversity-oriented approaches for interrogating T-cell receptor repertoire, ligand recognition, and function. Immunol. Rev. 250, 82–101 (2012).
Birnbaum, M. E. et al. Deconstructing the peptide-MHC specificity of T cell recognition. Cell 157, 1073–1087 (2014).
Mandl, J. N., Monteiro, J. P., Vrisekoop, N. & Germain, R. N. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity 38, 263–274 (2013).
Adams, J. J. et al. Structural interplay between germline interactions and adaptive recognition determines the bandwidth of TCR-peptide-MHC cross-reactivity. Nat. Immunol. 17, 87–94 (2015).
Schmid, D., Pypaert, M. & Münz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26, 79–92 (2007).
Sospedra, M. et al. Combining positional scanning peptide libraries, HLA-DR transfectants and bioinformatics to dissect the epitope spectrum of HLA class II cross-restricted CD4+T cell clones. J. Immunol. Methods 353, 93–101 (2010).
Judkowski, V. et al. GM-CSF production allows the identification of immunoprevalent antigens recognized by human CD4+ T cells following smallpox vaccination. PLoS ONE 6, e24091 (2011).
Wooldridge, L. et al. A single autoimmune T cell receptor recognizes more than a million different peptides. J. Biol. Chem. 287, 1168–1177 (2012).
Anderson, B., Park, B. J., Verdaguer, J., Amrani, A. & Santamaria, P. Prevalent CD8+ T cell response against one peptide/MHC complex in autoimmune diabetes. Proc. Natl Acad. Sci. USA 96, 9311–9316 (1999).
Wei, J. & Yewdell, J. W. News and views. Nat. Med. 23, 409–410 (2017).
Sadelain, M., Rivière, I. & Brentjens, R. J. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 3, 35–45 (2003).
Oxenius, A., Bachmann, M. F., Zinkernagel, R. M. & Hengartner, H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur. J. Immunol. 28, 390–400 (1998).
Valitutti, S., Müller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature 375, 148–151 (1995).
Kisielow, P. & Miazek, A. Positive selection of T cells: rescue from programmed cell death and differentiation require continual engagement of the T cell receptor. J. Exp. Med. 181, 1975–1984 (1995).
KirbergJ., Berns, A. & von Boehmer, H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex–encoded molecules. J. Exp. Med. 186, 1269 (1997).
Ignatowicz, L., Kappler, J. & Marrack, P. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell 84, 521–529 (1996).
Krishnamoorthy, G. et al. Myelin-specific T cells also recognize neuronal autoantigen in a transgenic mouse model of multiple sclerosis. Nat. Med. 15, 626–632 (2009).
Rosenthal, K. M. et al. Low 2-dimensional CD4 T cell receptor affinity for myelin sets in motion delayed response kinetics. PLoS ONE 7, e32562-11 (2012).
Subach, F. V. et al. Monomeric fluorescent timers that change color from blue to red report on cellular trafficking. Nat. Chem. Biol. 5, 118–126 (2009).
Bending, D. et al. A timer for analyzing temporally dynamic changes in transcription during differentiation in vivo. J. Cell Biol. 217, 2931–2950 (2018).
Ruedl, C., Bachmann, M. F. & Kopf, M. The antigen dose determines T helper subset development by regulation of CD40 ligand. Eur. J. Immunol. 30, 2056–2064 (2000).
Kataoka, T. et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J. Immunol. 156, 3678–3686 (1996).
Sahin, U. et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 547, 222–226 (2017).
Stagg, J. et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl Acad. Sci. USA 107, 1547–1552 (2010).
Tran, E. et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 350, 1387–1390 (2015).
Hayashi, H. et al. Molecular cloning and characterization of the gene encoding mouse melanoma antigen by cDNA library transfection. J. Immunol. 149, 1223–1229 (1992).
Brocker, T., Peter, A., Traunecker, A. & Karjalainen, K. New simplified molecular design for functional T cell receptor. Eur. J. Immunol. 23, 1435–1439 (1993).
Maher, J., Brentjens, R. J., Gunset, G., Rivière, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat. Biotechnol. 20, 70–75 (2002).
Jyothi, M. D., Flavell, R. A. & Geiger, T. L. Targeting autoantigen-specific T cells and suppression of autoimmune encephalomyelitis with receptor-modified T lymphocytes. Nat. Biotechnol. 20, 1215–1220 (2002).
Geiger, T. L., Leitenberg, D. & Flavell, R. A. The TCR ζ-chain immunoreceptor tyrosine-based activation motifs are sufficient for the activation and differentiation of primary T lymphocytes. J. Immunol. 162, 5931–5939 (1999).
Dotti, G., Gottschalk, S., Savoldo, B. & Brenner, M. K. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol. Rev. 257, 107–126 (2013).
Geiger, T. L., Nguyen, P., Leitenberg, D. & Flavell, R. A. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood 98, 2364–2371 (2001).
Zhang, T., He, X., Tsang, T. C. & Harris, D. T. SING: a novel strategy for identifying tumor-specific, CTL-recognized tumor antigens. FEBS. J. 18, 600–602 (2004).
Kracht, M. J. L. et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat. Med. 23, 501–507 (2017).
Dow, C. et al. Lymphocytic choriomeningitis virus infection yields overlapping CD4+ and CD8+ T-cell responses. J. Virol. 82, 11734–11741 (2008).
Masuda, K. et al. T cell lineage determination precedes the initiation of TCRβ gene rearrangement. J. Immunol. 179, 3699–3706 (2007).
Acknowledgements
We thank T. Schumacher (the Netherlands Cancer Institute, Amsterdam) for plasmids encoding mouse I-Ab MHC class II α and β chains, H. Kawamoto (Institute for Frontier Medical Sciences, Kyoto University, Japan) for the Tst-4/DLL1 cells, Ch. Münz and J. Ruehl (University Zurich) for the hTCCs, L. Tortola and J. Penninger (Institute of Molecular Biotechnology, Vienna, Austria) for the E0771 cell line, L. Ignatowicz (Medical College of Georgia, Augusta University, USA) for Ep splenocytes. We thank R. Martin for discussions, A. Schütz and M. Kisielow for help with cell sorting, L. Opitz for exome sequencing, E. Rosenwald and F. Ampenberger for technical assistance, and P. Nielsen and P. Kisielow for critical reading of the manuscript. Fluorescent timer proteins were designed at UCSD, and FT plasmids were acquired from Addgene (www.addgene.org). Financial support of M.K. by the Swiss National Science Foundation (grant no. 310030–163443) is appreciated.
Author information
Authors and Affiliations
Contributions
J.K. conceived the study, designed and performed experiments, analyzed data and wrote the manuscript. F.J.O. designed and performed experiments, analyzed and discussed data and reviewed the manuscript. M.K. analyzed and discussed data, corrected and provided critical feedback on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
On the basis of this study, a patent application was filed WO2016097334A1. The authors are founders of Tepthera.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Peptide-specific reactivity and sensitivity of the MCR2 sensor.
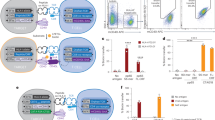
(a) Time course of peptide-specific NFAT activation (GFP reporter expression) in MCR2-OVA+H18.3.13 cells co-cultured with OT-II CD4+ T cells or SMARTA CD4+ T cells as controls. Histogram shows examples of NFAT activation measurements in MCR2-OVA+ cells at indicated time points (b) Sensitivity of detection of peptide-specific reporter cells. MCR2-OVA+ H18.3.13 cells were mixed with MCR2-gp61+ H18.3.13 cells at different ratios and NFAT activation was measured after co-culture with OT-II CD4+ T cells. The graph shows a linear (R>0.99) correlation between the percentage of GFP+ H18.3.13 cells and the percentage of cells carrying the MCR2-OVA. (c) A standard curve of reporter NFAT activation (blue-FT) in relation to the frequency of specific T cells is shown. The gray box represents standard deviation of the negative control. (d,e) Time course of MCR2-Ep downregulation in BEKO cells(d) and NFAT activation in MCR2-Ep+ reporter cells(e), co-cultured with CD4+ T cells from B6 or single-peptide mice(Ep). Values (d) show MCR2 levels depicted as percentage of mean fluorescence intensity at the start of co-culture. (f) NFAT activation in MCR2+ reporter cells carrying different peptides co-cultured (n = 3 co-cultures, mean ± sd) with hybridomas derived from SMARTA, Ep and 2D2 TCR transgenic mice. Data are representative of one (b,c,d), two (a,e) and three (f) independent experiments. Two-way ANOVA analysis with multiple pair-wise comparisons was done **** P ≤ 0.0001.
Supplementary Figure 2 Search for viral epitopes.
(a,b) CD4+ T cells from mice infected with LCMV were purified from the spleens 5 and 8 days p.i. and fused with α−β−BW5147 cells carrying an NFAT (GFP) reporter. Reactivity of the resulting hybridomas was determined by NFAT activation after co-culture with LCMV- or gp61-pulsed dendritic cells. Histograms (a) show examples of different types of reactivity (LCMV-open or gp61-filled dark gray) which are summarized in b. (c) A scheme of the cloning strategy for the construction of cDNA-derived peptide (CDP) libraries enriched for natural peptides. ORF – open reading frame dictating the natural protein sequence. (d) Cells transduced with the MCR2-CDP library were sorted into MCR2low, MCR2med and MCR2hi, and the length of the MCR-connected peptides was determined by PCR on the isolated DNA. No major difference in the length of the PCR products was observed, indicating that, in all fractions, mainly peptides between 15 and 50aa were present. (e) MCR2-LCMV+ 16.2c11 reporter cells were co-cultured with LCMV-reactive hybridomas (H2, H3, H14 and H30) of unknown peptide specificity. Activated reporter cells were sorted, expanded and co-cultured again with the corresponding hybridomas. Dot plots show example NFAT activation after two or three rounds of co-culture. (f) After the 3rd round of enrichment, single cells were sorted, expanded and their reactivity verified by co-culture with hybridomas (histogram shows example NFAT reactivity in reporter cells). The table on the right, shows frequencies of reactive clones. Peptide sequences from the MCR2 constructs, representing the dominant NP311 epitope and the new NP547 epitope are shown below. Figure represents data from one experiment.
Supplementary Figure 3 TCR dependent enrichment of reactive reporter cells.
Activation of MCR2-LCMV+ 16.2c11 cells after one, two and three rounds of enrichment by co-culture with hybridomas H3 and H4 (H4 is a TCR-negative control). Dot plots show NFAT activation, numbers indicate % cell in the gate. Figure represents data from one experiment.
Supplementary Figure 4 Clustal analysis of peptide homology.
Clustering analysis (done with Clustal Omega) of example H1- and C2-reactive peptide sequences, shown in blue and black, respectively. NP264 sequence is shown in red. Branch length in the right tree is proportional to the amount of inferred change between sequences.
Supplementary Figure 5 Reactivity of H1 and C2 hybridomas.
(a) T cell hybridomas H1 and C2 were stained with TCR Vbeta specific antibodies. All cells showed positive staining only with the TCR Vbeta14 antibody. (b) cDNA from hybridomas H1 and C2 was used to PCR and clone the TCRbeta chains using oligos specific for different TCRV beta chains. For both hybridomas, apart from the nonfunctional TCRb chain from the BW fusion partner (not shown) a functional TCRbeta containing the TCR Vbeta14 and TCRJbeta2–5 was cloned. A comparison of the protein sequences is shown, highlighting the different CDR3 regions. (c) Hybridomas carrying the NFAT-GFP reporter were co-cultured (n = 2 co-cultures) with BMDCs pulsed with different amounts of the indicated peptides (μg/ml, 90% purity). The graph shows NFAT activation (GFP). Data are representative of two independent experiments. (d) Half maximal effective concentration (EC50) was calculated by non-linear regression (curve fit) based on data from two experiments, mean ± sd are shown.
Supplementary Figure 6 Detection limit in a single co-culture.
Two hMCR2-DRB1.4-MP1103–120+ reporter clones were co-cultured with hTCC11–46 cells mixed with hTCC10–16 cells at different ratios. NFAT activation was measured after 8h (a) representative FACS dot plots, numbers indicate % NFAT+ cells and (b) statistical analysis of the results, (n = 3 co-cultures) mean ± sd are shown. One-way ANOVA analysis with multiple comparisons was done. * P < 0.02, **** P < 0.0001. nc, no co-culture.
Supplementary Figure 7 Sensitivity of the MCR system.
MCR2-gp61+ 16.2c11 reporter cells were spiked into MCR2-OVA + 16.2c11 reporter cells at different frequencies and NFAT activation (blue-FT) was measured after subsequent rounds of co-culture/enrichment with SMARTA T cell hybridoma cells (reporter to hybridoma ratio was 1/3(a) and 1/5(b). Last column (c) shows NFAT activation in co-cultures with SMARTA or OT-II T cell hybridoma cells after the last round of enrichment. Graphs show all live cells including reporter and hybridoma cells. Figure represents data from one experiment.
Supplementary information
Supplementary Figures 1–7 and Supplementary Table 1
Examples of cross-reactive artificial peptides present in the CDP library. They are derived mostly from UTRs, antisense (AS) reading frames, frame shifts or intronic sequences.
Rights and permissions
About this article
Cite this article
Kisielow, J., Obermair, FJ. & Kopf, M. Deciphering CD4+ T cell specificity using novel MHC–TCR chimeric receptors. Nat Immunol 20, 652–662 (2019). https://doi.org/10.1038/s41590-019-0335-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-019-0335-z
This article is cited by
-
High-throughput discovery of MHC class I- and II-restricted T cell epitopes using synthetic cellular circuits
Nature Biotechnology (2025)
-
Circulating tumor-reactive KIR+CD8+ T cells suppress anti-tumor immunity in patients with melanoma
Nature Immunology (2025)
-
Novel insights into TCR-T cell therapy in solid neoplasms: optimizing adoptive immunotherapy
Experimental Hematology & Oncology (2024)
-
The therapeutic potential of immunoengineering for systemic autoimmunity
Nature Reviews Rheumatology (2024)
-
De novo identification of CD4+ T cell epitopes
Nature Methods (2024)