Abstract
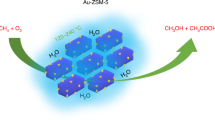
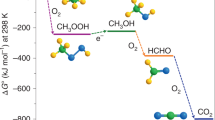
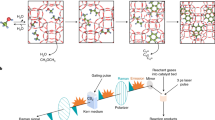
Anthropogenic methane emissions, particularly from diffuse and dilute sources, pose a significant challenge for oxidation and valorization as existing methane oxidation routes rely on high temperatures or pressures. Here we report the catalytic coupling of alcohol oxidase with the iron-modified ZSM-5 (Fe-ZSM-5) zeolite catalyst, creating a tandem methanotrophic system that partially oxidizes methane at ambient temperatures and pressures. Methane reacts at Fe-ZSM-5 to produce methanol, which is then oxidized at the enzyme to formaldehyde and hydrogen peroxide. The latter subsequently reacts back at Fe-ZSM-5 and oxidizes methane in a catalytic couple. We show that methane-to-formaldehyde selectivity can exceed 90% at room temperature. The generated formaldehyde was rapidly incorporated into a growing urea polymer, with a material growth rate exceeding 5.0 mg gcat−1 h−1, which matches or exceeds the growth rates of many methanotrophic organisms. This work presents a sustainable route for methane oxidation, driven by oxygen in the air under ambient conditions, producing high-value polymers and valorizing methane emission streams.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the Article and its Supplementary Information or from the corresponding author upon reasonable request.
References
Global Methane Tracker 2022 (IEA, 2022); https://www.iea.org/reports/global-methane-tracker-2022
Staniaszek, Z. et al. The role of future anthropogenic methane emissions in air quality and climate. npj Clim. Atmos. Sci. 5, 21 (2022).
Wuebbles, D. J. & Hayhoe, K. Atmospheric methane and global change. Earth Sci. Rev. 57, 177–210 (2002).
Allen, D. T. Methane emissions from natural gas production and use: reconciling bottom-up and top-down measurements. Curr. Opin. Chem. Eng. 5, 78–83 (2014).
Mansoor, R. & Tahir, M. Recent developments in natural gas flaring reduction and reformation to energy-efficient fuels: a review. Energy Fuels 35, 3675–3714 (2021).
Oonk, H. Efficiency of landfill gas collection for methane emission reduction. Greenhouse Gas. Meas. Manage. 2, 129–145 (2012).
Plant, G. et al. Inefficient and unlit natural gas flares both emit large quantities of methane. Science 377, 1566–1571 (2022).
Van Amstel, A. Methane. A review. J. Integr. Environ. Sci. 9, 5–30 (2012).
Turner, A. J., Frankenberg, C. & Kort, E. A. Interpreting contemporary trends in atmospheric methane. Proc. Natl Acad. Sci. USA 116, 2805–2813 (2019).
Jackson, R. B. et al. Atmospheric methane removal: a research agenda. Philos. Trans. R. Soc. A 379, 20200454 (2021).
Amaral, J. A., Ren, T. & Knowles, R. Atmospheric methane consumption by forest soils and extracted bacteria at different pH values. Appl. Environ. Microbiol. 64, 2397–2402 (1998).
Khider, M. L. K., Brautaset, T. & Irla, M. Methane monooxygenases: central enzymes in methanotrophy with promising biotechnological applications. World J. Microbiol. Biotechnol. 37, 72 (2021).
Strong, P. J., Xie, S. & Clarke, W. P. Methane as a resource: can the methanotrophs add value? Environ. Sci. Technol. 49, 4001–4018 (2015).
Kalyuzhnaya, M. G., Puri, A. W. & Lidstrom, M. E. Metabolic engineering in methanotrophic bacteria. Metab. Eng. 29, 142–152 (2015).
Hwang, I. Y. et al. Biological conversion of methane to chemicals and fuels: technical challenges and issues. Appl. Microbiol. Biotechnol. 102, 3071–3080 (2018).
Kerckhof, F. M. et al. From biogas and hydrogen to microbial protein through co-cultivation of methane and hydrogen oxidizing bacteria. Front. Bioeng. Biotechnol. 9, 733753 (2021).
Sahoo, K. K., Goswami, G. & Das, D. Biotransformation of methane and carbon dioxide into high-value products by methanotrophs: current state of art and future prospects. Front. Microbiol. 12, 636486 (2021).
Gęsicka, A., Oleskowicz-Popiel, P. & Łężyk, M. Recent trends in methane to bioproduct conversion by methanotrophs. Biotechnol. Adv. 53, 107861 (2021).
Strong, P. J., Kalyuzhnaya, M., Silverman, J. & Clarke, W. P. A methanotroph-based biorefinery: potential scenarios for generating multiple products from a single fermentation. Bioresour. Technol. 215, 314–323 (2016).
Conrado, R. J. & Gonzalez, R. Envisioning the bioconversion of methane to liquid fuels. Science 343, 621–623 (2014).
Haynes, C. A. & Gonzalez, R. Rethinking biological activation of methane and conversion to liquid fuels. Nat. Chem. Biol. 10, 331–339 (2014).
Liao, J. C., Mi, L., Pontrelli, S. & Luo, S. Fuelling the future: microbial engineering for the production of sustainable biofuels. Nat. Rev. Microbiol. 14, 288–304 (2016).
Yasin, M. et al. Microbial synthesis gas utilization and ways to resolve kinetic and mass-transfer limitations. Bioresour. Technol. 177, 361–374 (2015).
Khirsariya, P. & Mewada, R. K. Single step oxidation of methane to methanol—towards better understanding. Procedia Eng. 51, 409–415 (2013).
Dummer, N. F. et al. Methane oxidation to methanol. Chem. Rev. 123, 6359–6411 (2023).
Bryliakov, K. P. & Talsi, E. P. Active sites and mechanisms of bioinspired oxidation with H2O2, catalyzed by non-heme Fe and related Mn complexes. Coord. Chem. Rev. 276, 73–96 (2014).
York, A. P. E., Xiao, T. & Green, M. L. H. Brief overview of the partial oxidation of methane to synthesis gas. Top. Catal. 22, 345–358 (2003).
Lange, J.-P. Methanol synthesis: a short review of technology improvements. Catal. Today 64, 3–8 (2001).
Saunois, M., Jackson, R. B., Bousquet, P., Poulter, B. & Canadell, J. G. The growing role of methane in anthropogenic climate change. Environ. Res. Lett. 11, 120207 (2016).
Hammond, C. et al. Direct catalytic conversion of methane to methanol in an aqueous medium by using copper-promoted Fe-ZSM-5. Angew. Chem. Int. Ed. 51, 5129–5133 (2012).
Yang, N., Ren, Z., Yang, C., Wu, P. & Zeng, G. Direct oxidation of CH4 to HCOOH over extra-framework stabilized Fe@MFI catalyst at low temperature. Fuel 305, 121624 (2021).
Cheng, Q. et al. Maximizing active Fe species in ZSM-5 zeolite using organic-template-free synthesis for efficient selective methane oxidation. J. Am. Chem. Soc. 145, 5888–5898 (2023).
Hammond, C. et al. Catalytic and mechanistic insights of the low-temperature selective oxidation of methane over Cu-promoted Fe-ZSM-5. Chem. Eur. J. 18, 15735–15745 (2012).
Forde, M. M. et al. Partial oxidation of ethane to oxygenates using Fe- and Cu-containing ZSM-5. J. Am. Chem. Soc. 135, 11087–11099 (2013).
Szécsényi, Á., Li, G., Gascon, J. & Pidko, E. A. Mechanistic complexity of methane oxidation with H2O2 by single-site Fe/ZSM-5 catalyst. ACS Catal. 8, 7961–7972 (2018).
Puértolas, B., Hill, A. K., García, T., Solsona, B. & Torrente-Murciano, L. In-situ synthesis of hydrogen peroxide in tandem with selective oxidation reactions: a mini-review. Catal. Today 248, 115–127 (2015).
Wu, B. et al. Selective oxidation of methane to oxygenates using oxygen via tandem catalysis. Chem. Eur. J. 29, e202203057 (2023).
Reyes-De-Corcuera, J. I., Olstad, H. E. & García-Torres, R. Stability and stabilization of enzyme biosensors: the key to successful application and commercialization. Annu. Rev. Food Sci. Technol. 9, 293–322 (2018).
Deka, T. J., Osman, A. I., Baruah, D. C. & Rooney, D. W. Methanol fuel production, utilization, and techno-economy: a review. Environ. Chem. Lett. 20, 3525–3554 (2022).
Dumée, L. F. Circular materials and circular design—review on challenges towards sustainable manufacturing and recycling. Circ. Econ. Sustain. 2, 9–23 (2022).
Ravindran, L., Sreekala, M. S., Anil Kumar, S. & Thomas, S. A comprehensive review on phenol-formaldehyde resin-based composites and foams. Polym. Compos. 43, 8602–8621 (2022).
Nuryawan, A., Risnasari, I., Sucipto, T., Heri Iswanto, A. & Rosmala Dewi, R. Urea-formaldehyde resins: production, application, and testing. IOP Conf. Ser. Mater. Sci. Eng. 223, 012053 (2017).
Li, W., Zhu, X., Zhao, N. & Jiang, Z. Preparation and properties of melamine urea-formaldehyde microcapsules for self-healing of cementitious materials. Materials (Basel) 9, 152 (2016).
Nair, B. R. & Francis, D. J. Kinetics and mechanism of urea-formaldehyde reaction. Polymer (Guildf.) 24, 626–630 (1983).
Smythe, L. E. A kinetic study of the urea-formaldehyde reaction. J. Phys. Colloid Chem. 51, 369–378 (1947).
Sahm, H. Oxidation of formaldehyde by alcohol oxidase of Candida boidinii. Arch. Microbiol. 105, 179–181 (1975).
Steinhof, O., Kibrik, É. J., Scherr, G. & Hasse, H. Quantitative and qualitative 1H, 13C, and 15N NMR spectroscopic investigation of the urea-formaldehyde resin synthesis. Magn. Reson. Chem. 52, 138–162 (2014).
Liu, L.-Y. et al. Biological conversion of methane to polyhydroxyalkanoates: current advances, challenges, and perspectives. Environ. Sci. Ecotechnol. 2, 100029 (2020).
Pieja, A. J., Sundstrom, E. R. & Criddle, C. S. Cyclic, alternating methane and nitrogen limitation increases PHB production in a methanotrophic community. Bioresour. Technol. 107, 385–392 (2012).
Helm, J., Wendlandt, K.-D., Jechorek, M. & Stottmeister, U. Potassium deficiency results in accumulation of ultra-high molecular weight poly-β-hydroxybutyrate in a methane-utilizing mixed culture. J. Appl. Microbiol. 105, 1054–1061 (2008).
Gonçalves, C. et al. Impact of the synthesis procedure on urea-formaldehyde resins prepared by alkaline–acid process. Ind. Eng. Chem. Res. 58, 5665–5676 (2019).
Ding, Z., Ding, Z., Ma, T. & Zhang, H. Condensation reaction and crystallization of urea-formaldehyde resin during the curing process. Bioresources 15, 2924–2936 (2020).
Blanchette, C. D. et al. Printable enzyme-embedded materials for methane to methanol conversion. Nat. Commun. 7, 11900 (2016).
Guilbault, G. G., Danielsson, B., Mandenius, C. F. & Mosbach, K. Enzyme electrode and thermistor probes for determination of alcohols with alcohol oxidase. Anal. Chem. 55, 1582–1585 (1983).
Gonchar, M. V., Maidan, M. M., Moroz, O. M., Woodward, J. R. & Sibirny, A. A. Microbial O2- and H2O2-electrode sensors for alcohol assays based on the use of permeabilized mutant yeast cells as the sensitive bioelements. Biosens. Bioelectron. 13, 945–952 (1998).
Viña-Gonzalez, J., Gonzalez-Perez, D., Ferreira, P., Martinez, A. T. & Alcalde, M. Focused directed evolution of aryl-alcohol oxidase in Saccharomyces cerevisiae by using chimeric signal peptides. Appl. Environ. Microbiol. 81, 6451–6462 (2015).
Lundberg, D. J., Kim, J., Parviz, D. & Strano, M. S. Mitigation of ventilation air methane (VAM) using novel methanotrophic coating materials: a technical analysis. Environ. Res. Lett. 18, 114039 (2023).
Baranowski, C. J., Bahmanpour, A. M. & Kröcher, O. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): a review. Appl. Catal. B 217, 407–420 (2017).
Sadeghi, M., Hanifpour, F., Taheri, R., Javadian, H. & Ghasemi, M. Comparison of using formaldehyde and carboxy methyl chitosan in preparation of Fe3O4 superparamagnetic nanoparticles-chitosan hydrogel network: sorption behavior toward bovine serum albumin. Process Saf. Environ. Prot. 102, 119–128 (2016).
Rico-García, D. et al. Lignin-based hydrogels: synthesis and applications. Polymers 12, 81 (2020).
Merline, D. J., Vukusic, S. & Abdala, A. A. Melamine formaldehyde: curing studies and reaction mechanism. Polym. J. 45, 413–419 (2013).
Cao, M., Li, T., Liang, J. & Du, G. The influence of pH on the melamine-dimethylurea-formaldehyde co-condensations: a quantitative 13C-NMR study. Polymers (Basel) 9, 109 (2017).
Li, T., Cao, M., Zhang, B., Yang, L. & Du, G. Effects of molar ratio and pH on the condensed structures of melamine-formaldehyde polymers. Materials (Basel) 11, 2571 (2018).
Yu, T. et al. Identifying key mononuclear Fe species for low-temperature methane oxidation. Chem. Sci. 12, 3152–3160 (2021).
Beach, C. A. et al. Quantitative carbon detector for enhanced detection of molecules in foods, pharmaceuticals, cosmetics, flavors, and fuels. Analyst 141, 1627–1632 (2016).
Lundberg, D. J. et al. Universal kinetic mechanism describing CO2 photoreductive yield and selectivity for semiconducting nanoparticle photocatalysts. J. Am. Chem. Soc. 144, 13623–13633 (2022).
Youkhana, E. Q., Feltis, B., Blencowe, A. & Geso, M. Titanium dioxide nanoparticles as radiosensitisers: an in vitro and phantom-based study. Int. J. Med. Sci. 14, 602–614 (2017).
Silmore, K. S., Gong, X., Strano, M. S. & Swan, J. W. High-resolution nanoparticle sizing with maximum a posteriori nanoparticle tracking analysis. ACS Nano 13, 3940–3952 (2019).
Yashnik, S., Boltenkov, V., Babushkin, D., Taran, O. & Parmon, V. Methane oxidation by H2O2 over different Cu-species of Cu-ZSM-5 catalysts. Top. Catal. 63, 203–221 (2020).
Yashnik, S. A., Boltenkov, V. V., Babushkin, D. E., Surovtsova, T. A. & Parmon, V. N. Liquid-phase methane peroxidation in the presence of Cu-ZSM-5: effect of modification with palladium. Kinet. Catal. 63, 555–568 (2022).
Xu, J. et al. Continuous selective oxidation of methane to methanol over Cu- and Fe-modified ZSM-5 catalysts in a flow reactor. Catal. Today 270, 93–100 (2016).
Xiao, P., Wang, Y., Nishitoba, T., Kondo, J. N. & Yokoi, T. Selective oxidation of methane to methanol with H2O2 over an Fe-MFI zeolite catalyst using sulfolane solvent. Chem. Commun. 55, 2896–2899 (2019).
Yuan, Q., Deng, W., Zhang, Q. & Wang, Y. Osmium-catalyzed selective oxidations of methane and ethane with hydrogen peroxide in aqueous medium. Adv. Synth. Catal. 349, 1199–1209 (2007).
Taran, O. P. et al. Formic acid production via methane peroxide oxidation over oxalic acid activated Fe-MFI catalysts. Top. Catal. 62, 491–507 (2019).
Sun, S. et al. Lanthanum modified Fe-ZSM-5 zeolites for selective methane oxidation with H2O2. Catal. Sci. Technol. 11, 8052–8064 (2021).
Lewis, R. J. et al. The direct synthesis of H2O2 and selective oxidation of methane to methanol using HZSM-5 supported AuPd catalysts. Catal. Lett. 149, 3066–3075 (2019).
Aizat, A. G., Paiman, B., Lee, S. H. & Zaidon, A. Physico-mechanical properties and formaldehyde emission of rubberwood particleboard made with UF resin admixed with ammonium and aluminium-based hardeners. Pertanika J. Sci. Technol. 27, 473–488 (2019).
Park, B.-D., Frihart, C. R., Yu, Y. & Singh, A. P. Hardness evaluation of cured urea–formaldehyde resins with different formaldehyde/urea mole ratios using nanoindentation method. Eur. Polym. J. 49, 3089–3094 (2013).
Acknowledgements
We would like to acknowledge the support of funding from the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences (Grant DE-FG02-08ER46488). SEM, TEM, ICP-OES and XPS were carried out in part through the use of MIT.nano Characterization Facilities. We thank the MIT Department of Chemistry Instrumentation Facility for the use of their NMR spectrometers. This material is based on work sponsored in part by the US Army DEVCOM ARL Army Research Office through the MIT Institute for Soldier Nanotechnologies under Cooperative Agreement number W911NF-23-2-0121. We thank Y.-M. Jo and M. Dinca for the Brunauer–Emmett–Teller measurement of the ZSM-5 catalysts. We thank R. Zhu and Y. Roman for the ultraviolet-visible measurement of the ZSM-5 catalysts.
Author information
Authors and Affiliations
Contributions
M.S.S. supervised the project. M.S.S. and D.J.L. conceived the idea. D.J.L. and J.K. designed and carried out the experiments. Y.-M.T. carried out the TEM and TGA analyses. C.L.R. carried out the TEM analyses and methane oxidation reactions. All authors discussed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Damien Debecker, Richard Lewis, Feng-Shou Xiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–13, Methods, Tables 1–13 and Figs. 1–55.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lundberg, D.J., Kim, J., Tu, YM. et al. Concerted methane fixation at ambient temperature and pressure mediated by an alcohol oxidase and Fe-ZSM-5 catalytic couple. Nat Catal 7, 1359–1371 (2024). https://doi.org/10.1038/s41929-024-01251-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01251-z