Abstract
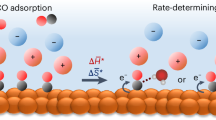
The electrochemical reduction of CO2 is sensitive to the microenvironment surrounding catalytic active sites. Although the impact of changing electrolyte composition on rates has been studied intensively in aqueous electrolytes, less is known about the influence of the electrochemical environment in non-aqueous solvents. Here we demonstrate that organic alkylammonium cations influence catalytic performance in non-aqueous media and describe a physical model that rationalizes these observations. Using results from kinetic, spectroscopic and computational techniques, we argue that the strength of the electric field at the catalyst surface is sensitive to the molecular identity of the organic cation in the electrolyte. This is true irrespective of solvent, electrolyte ionic strength or electrolyte anion. Our results suggest that changes in the interfacial electric field strength can be attributed to differences in the cation–electrode distance. Changes in the electric field strength affect CO formation rates as they modify the energetics of the kinetically relevant CO2 activation step.

This is a preview of subscription content, access via your institution
Access options








Similar content being viewed by others
Data availability
Computational datasets are available at the UCPH repository: https://sid.erda.dk/sharelink/hxqah2G0De. This includes all trajectory files from both relaxation and molecular dynamics simulations and covers initial and final configurations. Experimental datasets can be obtained from the corresponding author upon reasonable request.
References
Hori, Y. in Modern Aspects of Electrochemistry Vol. 42, 1st edn (eds Vayenas, C. G. et al.) 89–189 (Springer, 2008).
Stephens, I. E. L. et al. 2022 roadmap on low temperature electrochemical CO2 reduction. J. Phys. Energy 4, 042003 (2022).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Resasco, J. & Bell, A. T. Electrocatalytic CO2 reduction to fuels: progress and opportunities. Trends Chem. 2, 825–836 (2020).
König, M., Vaes, J., Klemm, E. & Pant, D. Solvents and supporting electrolytes in the electrocatalytic reduction of CO2. iScience 19, 135–160 (2019).
Govindarajan, N., Xu, A. & Chan, K. How pH affects electrochemical processes. Science 375, 379–380 (2022).
Waegele, M. M., Gunathunge, C. M., Li, J. & Li, X. How cations affect the electric double layer and the rates and selectivity of electrocatalytic processes. J. Chem. Phys. 151, 160902 (2019).
Potts, D. S., Bregante, D. T., Adams, J. S., Torres, C. & Flaherty, D. W. Influence of solvent structure and hydrogen bonding on catalysis at solid–liquid interfaces. Chem. Soc. Rev. 50, 12308–12337 (2021).
Li, G., Wang, B. & Resasco, D. E. Water-mediated heterogeneously catalyzed reactions. ACS Catal. 10, 1294–1309 (2020).
Deacon-Price, C., Da Silva, A. H. M., Santana, C. S., Koper, M. T. M. & Garcia, A. C. Solvent effect on electrochemical CO2 reduction reaction on nanostructured copper electrodes. J. Phys. Chem. C 127, 14518–14527 (2023).
Bender, J. T. et al. Understanding cation effects on the hydrogen evolution reaction. ACS Energy Lett. 8, 657–665 (2023).
Govindarajan, N., Kastlunger, G., Heenen, H. H. & Chan, K. Improving the intrinsic activity of electrocatalysts for sustainable energy conversion: where are we and where can we go? Chem. Sci. 13, 14–26 (2022).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Resasco, J. et al. Promoter effects of alkali metal cations on the electrochemical reduction of carbon dioxide. J. Am. Chem. Soc. 139, 11277–11287 (2017).
Ringe, S. et al. Understanding cation effects in electrochemical CO2 reduction. Energy Environ. Sci. 12, 3001–3014 (2019).
Gunathunge, C. M., Ovalle, V. J. & Waegele, M. M. Probing promoting effects of alkali cations on the reduction of CO at the aqueous electrolyte/copper interface. Phys. Chem. Chem. Phys. 19, 30166–30172 (2017).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Resasco, J., Lum, Y., Clark, E., Zeledon, J. Z. & Bell, A. T. Effects of anion identity and concentration on electrochemical reduction of CO2. ChemElectroChem 5, 1064–1072 (2018).
Murata, A. & Hori, Y. Product selectivity affected by cationic species in electrochemical reduction of CO2 and CO at a Cu electrode. BCSJ 64, 123–127 (1991).
Kas, R., Kortlever, R., Yılmaz, H., Koper, M. T. M. & Mul, G. Manipulating the hydrocarbon selectivity of copper nanoparticles in CO2 electroreduction by process conditions. Chem. Electro Chem. 2, 354–358 (2015).
Marcandalli, G., Monteiro, M. C. O., Goyal, A. & Koper, M. T. M. Electrolyte effects on CO2 electrochemical reduction to CO. Acc. Chem. Res. 55, 1900–1911 (2022).
Ovalle, V. J., Hsu, Y.-S., Agrawal, N., Janik, M. J. & Waegele, M. M. Correlating hydration free energy and specific adsorption of alkali metal cations during CO2 electroreduction on Au. Nat. Catal. 5, 624–632 (2022).
Li, J., Li, X., Gunathunge, C. M. & Waegele, M. M. Hydrogen bonding steers the product selectivity of electrocatalytic CO reduction. Proc. Natl Acad. Sci. USA 116, 9220–9229 (2019).
Haynes, L. V. & Sawyer, D. T. Electrochemistry of carbon dioxide in dimethyl sulfoxide at gold and mercury electrodes. Anal. Chem. 39, 332–338 (1967).
Chu, A. T., Jung, O., Toh, W. L. & Surendranath, Y. Organic non-nucleophilic electrolyte resists carbonation during selective CO2 electroreduction. J. Am. Chem. Soc. 145, 9617–9623 (2023).
Fogg, P. & Clever, L. (eds) Carbon Dioxide in Non-Aqueous Solvents at Pressures Less Than 200 KPA (Pergamon, 2017).
Ikeda, S., Takagi, T. & Ito, K. Selective formation of formic acid, oxalic acid, and carbon monoxide by electrochemical reduction of carbon dioxide. Bull. Chem. Soc. Jpn 60, 2517–2522 (1987).
Ito, K., Ikeda, S., Yamauchi, N., Iida, T. & Takagi, T. Electrochemical reduction products of carbon dioxide at some metallic electrodes in non-aqueous electrolytes. Bull. Chem. Soc. Jpn 58, 3027–3028 (1985).
Dubouis, N. et al. The fate of water at the electrochemical interfaces: electrochemical behavior of free water versus coordinating water. J. Phys. Chem. Lett. 9, 6683–6688 (2018).
Bagger, A., Christensen, O., Ivaništšev, V. & Rossmeisl, J. Catalytic CO2/CO reduction: gas, aqueous, and aprotic phases. ACS Catal. 12, 2561–2568 (2022).
Weng, S., Toh, W. L. & Surendranath, Y. Weakly coordinating organic cations are intrinsically capable of supporting CO2 reduction catalysis. J. Am. Chem. Soc. 145, 16787–16795 (2023).
Malkani, A. S. et al. Understanding the electric and non-electric field components of the cation effect on the electrochemical CO reduction reaction. Sci. Adv. 6, eabd2569 (2020).
Chen, L. D., Urushihara, M., Chan, K. & Nørskov, J. K. Electric field effects in electrochemical CO2 reduction. ACS Catal. 6, 7133–7139 (2016).
Mairegger, T. et al. Electroreduction of CO2 in a non-aqueous electrolyte—the generic role of acetonitrile. ACS Catal. 13, 5780–5786 (2023).
Foley, J. K., Korzeniewski, C. & Pons, S. Anodic and cathodic reactions in acetonitrile/tetra-n-butylammonium tetrafluoroborate: an electrochemical and infrared spectroelectrochemical study. Can. J. Chem. 66, 201–206 (1988).
Figueiredo, M. C., Ledezma-Yanez, I. & Koper, M. T. M. In situ spectroscopic study of CO2 electroreduction at copper electrodes in acetonitrile. ACS Catal. 6, 2382–2392 (2016).
Tomita, Y. & Hori, Y. in Studies in Surface Science and Catalysis Vol. 114 (eds Inui, T. et al.) 581–584 (Elsevier, 1998).
Shi, J. et al. Electrochemical reduction of CO2 into CO in tetrabutylammonium perchlorate/propylene carbonate: water effects and mechanism. Electrochim. Acta 240, 114–121 (2017).
Berto, T. C., Zhang, L., Hamers, R. J. & Berry, J. F. Electrolyte dependence of CO2 electroreduction: tetraalkylammonium ions are not electrocatalysts. ACS Catal. 5, 703–707 (2015).
Cencer, M. M. et al. Interactions of CO2 anion radicals with electrolyte environments from first-principles simulations. ACS Omega 7, 18131–18138 (2022).
König, M., Vaes, J., Pant, D. & Klemm, E. Effect of solvents on aprotic CO2 reduction: a study on the role of CO2 mass transport in the product selectivity between oxalate and carbon monoxide. J. Phys. Chem. C 127, 18159–18166 (2023).
Liu, B., Guo, W. & Gebbie, M. A. Tuning ionic screening to accelerate electrochemical CO2 reduction in ionic liquid electrolytes. ACS Catal. 12, 9706–9716 (2022).
Wuttig, A., Yoon, Y., Ryu, J. & Surendranath, Y. Bicarbonate is not a general acid in Au-catalyzed CO2 electroreduction. J. Am. Chem. Soc. 139, 17109–17113 (2017).
Wuttig, A., Yaguchi, M., Motobayashi, K., Osawa, M. & Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl Acad. Sci. USA 113, E4585–93 (2016).
Gomes, R. J. et al. Probing electrolyte influence on CO2 reduction in aprotic solvents. J. Phys. Chem. C. 126, 13595–13606 (2022).
Hansen, H. A., Varley, J. B., Peterson, A. A. & Nørskov, J. K. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO. J. Phys. Chem. Lett. 4, 388–392 (2013).
Ringe, S. The importance of a charge transfer descriptor for screening potential CO2 reduction electrocatalysts. Nat. Commun. 14, 2598 (2023).
Ringe, S. et al. Double layer charging driven carbon dioxide adsorption limits the rate of electrochemical carbon dioxide reduction on gold. Nat. Commun. 11, 33 (2020).
Alsunni, Y. A., Alherz, A. W. & Musgrave, C. B. Electrocatalytic reduction of CO2 to CO over Ag(110) and Cu(211) modeled by grand-canonical density functional theory. J. Phys. Chem. C 125, 23773–23783 (2021).
Rosen, J. et al. Mechanistic insights into the electrochemical reduction of CO2 to CO on nanostructured Ag surfaces. ACS Catal. 5, 4293–4299 (2015).
Clark, E. L. et al. Influence of atomic surface structure on the activity of Ag for the electrochemical reduction of CO2 to CO. ACS Catal. 9, 4006–4014 (2019).
Amatore, C. & Saveant, J. M. Mechanism and kinetic characteristics of the electrochemical reduction of carbon dioxide in media of low proton availability. J. Am. Chem. Soc. 103, 5021–5023 (1981).
Noh, S., Cho, Y. J., Zhang, G. & Schreier, M. Insight into the role of entropy in promoting electrochemical CO2 reduction by imidazolium cations. J. Am. Chem. Soc. 145, 27657–27663 (2023).
Guo, W., Liu, B. & Gebbie, M. A. Suppressing co-ion generation via cationic proton donors to amplify driving forces for electrochemical CO2 reduction. J. Phys. Chem. C 127, 14243–14254 (2023).
Deng, W., Zhang, P., Seger, B. & Gong, J. Unraveling the rate-limiting step of two-electron transfer electrochemical reduction of carbon dioxide. Nat. Commun. 13, 803 (2022).
Li, G., Wang, B. & Resasco, D. E. Solvent effects on catalytic reactions and related phenomena at liquid-solid interfaces. Surf. Sci. Rep. 76, 100541 (2021).
Linic, S. & Barteau, M. A. On the mechanism of Cs promotion in ethylene epoxidation on Ag. J. Am. Chem. Soc. 126, 8086–8087 (2004).
Lang, N. D., Holloway, S. & Nørskov, J. K. Electrostatic adsorbate–adsorbate interactions: the poisoning and promotion of the molecular adsorption reaction. Surf. Sci. 150, 24–38 (1985).
Roth, J. D. & Weaver, M. J. Role of double-layer cation on the potential-dependent stretching frequencies and binding geometries of carbon monoxide at platinum–non-aqueous interfaces. Langmuir 8, 1451–1458 (1992).
Fried, S. D. & Boxer, S. G. Measuring electric fields and non-covalent interactions using the vibrational Stark effect. Acc. Chem. Res. 48, 998–1006 (2015).
Lambert, D. K. Vibrational Stark effect of CO on Ni(100), and CO in the aqueous double layer: experiment, theory, and models. J. Chem. Phys. 89, 3847–3860 (1988).
Cuesta, A. Measurement of the surface charge density of CO-saturated Pt(111) electrodes as a function of potential: the potential of zero charge of Pt(111). Surf. Sci. 572, 11–22 (2004).
Anderson, M. R. & Huang, J. The influence of cation size upon the infrared spectrum of carbon monoxide adsorbed on platinum electrodes. J. Electroanal. Chem. Interfacial Electrochem. 318, 335–347 (1991).
Tao, F. et al. Break-up of stepped platinum catalyst surfaces by high CO coverage. Science 327, 850–853 (2010).
Mamatkulov, M. & Filhol, J.-S. An study of electrochemical vs. electromechanical properties: the case of CO adsorbed on a Pt(111) surface. Phys. Chem. Chem. Phys. 13, 7675 (2011).
Dimakis, N., Navarro, N. E., Mion, T. & Smotkin, E. S. Carbon monoxide adsorption coverage study on platinum and ruthenium surfaces. J. Phys. Chem. C 118, 11711–11722 (2014).
Suydam, I. T. & Boxer, S. G. Vibrational Stark effects calibrate the sensitivity of vibrational probes for electric fields in proteins. Biochemistry 42, 12050–12055 (2003).
Clark, M. L. et al. CO2 reduction catalysts on gold electrode surfaces influenced by large electric fields. J. Am. Chem. Soc. 140, 17643–17655 (2018).
Staffa, J. K. et al. Determination of the local electric field at Au/SAM interfaces using the vibrational Stark effect. J. Phys. Chem. C 121, 22274–22285 (2017).
Ge, A. et al. Interfacial structure and electric field probed by in situ electrochemical vibrational Stark effect spectroscopy and computational modeling. J. Phys. Chem. C 121, 18674–18682 (2017).
Bhattacharyya, D. et al. Vibrational Stark shift spectroscopy of catalysts under the influence of electric fields at electrode–solution interfaces. Chem. Sci. 12, 10131–10149 (2021).
Zhao, K. et al. Action at a distance: organic cation induced long range organization of interfacial water enhances hydrogen evolution and oxidation kinetics. Chem. Sci. 14, 11076–11087 (2023).
Lambert, D. K. Vibrational Stark effect of adsorbates at electrochemical interfaces. Electrochim. Acta 41, 623–630 (1996).
Chang, S. C., Jiang, X., Roth, J. D. & Weaver, M. J. Influence of potential on metal–adsorbate structure: solvent-independent nature of infrared spectra for platinum(111) carbon monoxide. J. Phys. Chem. 95, 5378–5382 (1991).
Zhu, Q., Wallentine, S. K., Deng, G.-H., Rebstock, J. A. & Baker, L. R. The solvation-induced Onsager reaction field rather than the double-layer field controls CO2 reduction on gold. JACS Au 2, 472–482 (2022).
Levell, Z. et al. Emerging atomistic modeling methods for heterogenous electrocatalysis. Chem. Rev. 124, 8620–8656 (2024).
Resasco, J. et al. Enhancing the connection between computation and experiments in electrocatalysis. Nat. Catal. 5, 374–381 (2022).
Montoya, J. H., Shi, C., Chan, K. & Nørskov, J. K. Theoretical insights into a CO dimerization mechanism in CO2 electroreduction. J. Phys. Chem. Lett. 6, 2032–2037 (2015).
Gauthier, J. A. et al. Challenges in modeling electrochemical reaction energetics with polarizable continuum models. ACS Catal. 9, 920–931 (2019).
Zhao, S.-F., Horne, M., Bond, A. M. & Zhang, J. Is the imidazolium cation a unique promoter for electrocatalytic reduction of carbon dioxide? J. Phys. Chem. C 120, 23989–24001 (2016).
Clark, E. L. et al. Standards and protocols for data acquisition and reporting for studies of the electrochemical reduction of carbon dioxide. ACS Catal. 8, 6560–6570 (2018).
Lobaccaro, P. et al. Effects of temperature and gas–liquid mass transfer on the operation of small electrochemical cells for the quantitative evaluation of CO2 reduction electrocatalysts. Phys. Chem. Chem. Phys. 18, 26777–26785 (2016).
Chae, K. J. et al. Mass transport through a proton exchange membrane (Nafion) in microbial fuel cells. Energy Fuels 22, 169–176 (2008).
Delley, M. F., Nichols, E. M. & Mayer, J. M. Electrolyte cation effects on interfacial acidity and electric fields. J. Phys. Chem. C 126, 8477–8488 (2022).
Enkovaara, J. et al. Electronic structure calculations with GPAW: a real-space implementation of the projector augmented-wave method. J. Phys. Condens. Matter 22, 253202 (2010).
Mortensen, J. J., Hansen, L. B. & Jacobsen, K. W. Real-space grid implementation of the projector augmented wave method. Phys. Rev. B 71, 035109 (2005).
Hjorth Larsen, A. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Hammer, B., Hansen, L. B. & Norskov, J. K. Improved adsorption energetics within density-functional theory using revised Perdew–Burke–Ernzerhof functionals. Phys. Rev. B 59, 7413–7421 (1999).
Berendsen, H. J. C., Postma, J. P. M., Van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 81, 3684–3690 (1984).
Acknowledgements
J.M. gratefully acknowledges the support of ExxonMobil Corporation through the Future Leaders Academy Ph.D. Fellowship. Findings and conclusions are those of the author and do not reflect the views of ExxonMobil Corporation. J.F.B and J. Resasco gratefully acknowledge support from the Robert A. Welch Foundation (Grants F-1945 and F-2076, respectively). We thank A. Kennedy from the University of Texas at Austin Dept. of Chemistry’s Glass Shop for the construction of electrochemistry glass cell components and D. Davies for assistance with graphic design. J.T.B. gratefully acknowledges the support of the National Science Foundation Graduate Research Fellowship Program (NSF-GRFP) under Grant Nos. DGE-1610403 and DGE-2137420. A.S.P and J. Rossmeisl thank the Center for High Entropy Alloy (CHEAC) funded by the Danish National Research Foundation (DNRF 149) and the Villum Foundation through the Villum Center for the Science of Sustainable Fuels and Chemicals (#9444) for funding this work.
Author information
Authors and Affiliations
Contributions
J.M. conducted all electrochemical experiments and wrote the paper. J.T.B. collected all the vibrational spectroscopy data. A.S.P. performed all the computational calculations and J. Rossmeisl guided the computational efforts. L.C. synthesized and characterized the asymmetric quaternary cations. J. Resasco conceptualized the paper. J.F.B and J. Resasco guided the work. All authors contributed to the discussion, review and editing of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–64; Tables 1–5 and Notes 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McGregor, JM., Bender, J.T., Petersen, A.S. et al. Organic electrolyte cations promote non-aqueous CO2 reduction by mediating interfacial electric fields. Nat Catal 8, 79–91 (2025). https://doi.org/10.1038/s41929-024-01278-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-024-01278-2
This article is cited by
-
Clarifying cation control
Nature Catalysis (2025)