Abstract
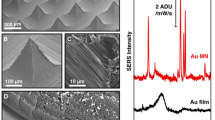
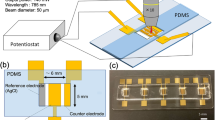
Electrochemical deposition has been widely used to prepare conformal coatings but has rarely been used to design well-defined micro/nanostructures. Here we report electrochemical synthesis of complex, hierarchical inorganic microarchitectures simply via programming the applied potential waveforms. We identify two distinct electrochemical growth modes—the stacking mode and the flattening mode—under different potential waveforms. We demonstrate how these growth modes can work individually or cooperatively to design previously inaccessible microarchitectures. Each specific potential waveform corresponds to a specific microarchitecture, allowing us to prepare a rich library of microarchitectures. The designed microarchitectures can be converted into other materials by simple redox-potential-driven chemical reactions. We preliminarily studied the applications of converted nanoporous silver microscale torpedoes as high-performance surface-enhanced Raman spectroscopy (SERS) sensing substrates. The reported method opens up a new concept to design complex inorganic microarchitectures with promising applications in metamaterials, chemically or magnetically propelled microrobotics, and miniaturized devices.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Noorduin, W. L., Grinthal, A., Mahadevan, L. & Aizenberg, J. Rationally designed complex, hierarchical microarchitectures. Science 340, 832–837 (2013).
Luo, Z. et al. Atomic gold–enabled three-dimensional lithography for silicon mesostructures. Science 348, 1451–1455 (2015).
Vukusic, P. & Sambles, J. R. Photonic structures in biology. Nature 424, 852–855 (2003).
Miskin, M. Z. et al. Electronically integrated, mass-manufactured, microscopic robots. Nature 584, 557–561 (2020).
Xu, S. et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 344, 70–74 (2014).
Zhu, Z., Ng, D. W. H., Park, H. S. & McAlpine, M. C. 3D-printed multifunctional materials enabled by artificial-intelligence-assisted fabrication technologies. Nat. Rev. Mater. 6, 27–47 (2020).
Madou, M. J. Fundamentals of Microfabrication: The Science of Miniaturization (CRC Press, 2002).
Maruo, S., Nakamura, O. & Kawata, S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Opt. Lett. 22, 132–134 (1997).
Melchels, F. P. W., Feijen, J. & Grijpma, D. W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 31, 6121–6130 (2010).
Sun, Y. & Xia, Y. Shape-controlled synthesis of gold and silver nanoparticles. Science 298, 2176–2179 (2002).
Lee, H.-E. et al. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 556, 360–365 (2018).
Ben-Moshe, A. et al. The chain of chirality transfer in tellurium nanocrystals. Science 372, 729–733 (2021).
Tsivion, D., Schvartzman, M., Popovitz-Biro, R., von Huth, P. & Joselevich, E. Guided growth of millimeter-long horizontal nanowires with controlled orientations. Science 333, 1003–1007 (2011).
Lin, Q.-Y. et al. Building superlattices from individual nanoparticles via template-confined DNA-mediated assembly. Science 359, 669–672 (2018).
Dick, K. A. et al. Synthesis of branched ‘nanotrees’ by controlled seeding of multiple branching events. Nat. Mater. 3, 380–384 (2004).
Liu, M. et al. Enhanced electrocatalytic CO2 reduction via field-induced reagent concentration. Nature 537, 382–386 (2016).
Andricacos, P. C., Uzoh, C., Dukovic, J. O., Horkans, J. & Deligianni, H. Damascene copper electroplating for chip interconnections. IBM J. Res. Dev. 42, 567–574 (1998).
Oskam, G., Long, J., Natarajan, A. & Searson, P. Electrochemical deposition of metals onto silicon. J. Phys. D 31, 1927 (1998).
Mahenderkar, N. K. et al. Epitaxial lift-off of electrodeposited single-crystal gold foils for flexible electronics. Science 355, 1203–1206 (2017).
Zach, M. P., Ng, K. H. & Penner, R. M. Molybdenum nanowires by electrodeposition. Science 290, 2120–2123 (2000).
Tian, N., Zhou, Z.-Y., Sun, S.-G., Ding, Y. & Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 316, 732–735 (2007).
Siegfried, M. J. & Choi, K. S. Electrochemical crystallization of cuprous oxide with systematic shape evolution. Adv. Mater. 16, 1743–1746 (2004).
Zheng, J. et al. Reversible epitaxial electrodeposition of metals in battery anodes. Science 366, 645–648 (2019).
Lopez Teijelo, M., Vilche, J. R. & Arvia, A. J. The electroformation and electroreduction of anodic films formed on silver in 0.1 M sodium hydroxide in the potential range of the Ag/Ag2O couple. J. Electroanal. Chem. Interf. Electrochem. 162, 207–224 (1984).
McMillan, J. Higher oxidation states of silver. Chem. Rev. 62, 65–80 (1962).
Zamora-Garcia, I. R. et al. Thermodynamic and electrochemical study on the mechanism of formation of Ag(OH)4− in alkaline media. Electrochim. Acta 111, 268–274 (2013).
Savinova, E. R. et al. On the mechanism of Ag(111) sub-monolayer oxidation: a combined electrochemical, in situ SERS and ex situ XPS study. Electrochim. Acta 46, 175–183 (2000).
Cui, L. F., Ying, Y. L., Yu, R. J., Ma, H., Hu, P. & Long, Y. T. In situ characterization of oxygen evolution electrocatalysis of silver salt oxide on a wireless nanopore electrode. Anal. Chem. 94, 15033–15039 (2022).
Buck, R. P. Kinetics of bulk and interfacial ionic motion: microscopic bases and limits for the Nernst–Planck equation applied to membrane systems. J. Membrane Sci. 17, 1–62 (1984).
Wang, Y. et al. Electrocarving during electrodeposition growth. Adv. Mater. 30, e1805686 (2018).
Hoyle, B. D. & Costerton, J. W. Bacterial resistance to antibiotics: the role of biofilms. Prog. Drug Res. 37, 91–105 (1991).
Hendrikse, H. C. et al. Shape-preserving chemical conversion of architected nanocomposites. Adv. Mater. 32, e2003999 (2020).
Wang, X., Huang, S.-C., Hu, S., Yan, S. & Ren, B. Fundamental understanding and applications of plasmon-enhanced Raman spectroscopy. Nat. Rev. Phys. 2, 253–271 (2020).
Chen, X., Ding, Q., Bi, C., Ruan, J. & Yang, S. Lossless enrichment of trace analytes in levitating droplets for multiphase and multiplex detection. Nat. Commun. 13, 7807 (2022).
Michaels, A. M., Jiang, J. & Brus, L. Ag nanocrystal junctions as the site for surface-enhanced Raman scattering of single rhodamine 6G molecules. J. Phys. Chem. B 104, 11965–11971 (2000).
Li, J.-F., Zhang, Y.-J., Ding, S.-Y., Panneerselvam, R. & Tian, Z.-Q. Core–shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 117, 5002–5069 (2017).
Xu, H., Aizpurua, J., Käll, M. & Apell, P. Electromagnetic contributions to single-molecule sensitivity in surface-enhanced Raman scattering. Phys. Rev. E 62, 4318–4324 (2000).
Bard, A., Oarsons, R. & Jordan, J. Standard Potentials in Aqueous Solution (Routledge, 1985).
Acknowledgements
We acknowledge funding support from the Key R&D Program of Zhejiang Province (2023C01088), the National Natural Science Foundation of China (52273233 and 51971200), the Zhejiang Provincial Natural Science Foundation of China (LR19E010001) and the Open Research Program of Key Laboratory of 3D Micro/Nano Fabrication and Characterization of Zhejiang Province, Westlake University. Part of the work was conducted in the ZJU micro-nanofabrication center.
Author information
Authors and Affiliations
Contributions
S.Y. and L.Z. conceived the idea and designed the study. L.Z., Y.W. and N.A. carried out the materials synthesis and characterizations. S.J. and Z.H. performed the phase-field simulations. L.Z., M.Y., X.Z., Z.H. and S.Y. analysed the data. L.Z., M.Y., Z.H. and S.Y. wrote the paper. All authors contributed to the revision of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Domenica Tonelli, Yexiang Tong and Jon Ustarroz for their contribution to the peer review of this work. Primary Handling Editor: Alexandra Groves, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary discussion, captions for Videos 1–5 and Figs. 1–34.
Supplementary Video 1
The microarchitecture design concept: the morphology evolvement of the microarchitectures as the potential waveform changes.
Supplementary Video 2
The change of the ion concentration distribution and the corresponding morphology change when applying 0.18 V to a preformed micropyramid and when the potential is increased from 0.18 V to 0.22 V simulated by the phase-field method.
Supplementary Video 3
The change of the ion concentration distribution and the corresponding morphology change when applying 0.12 V to a preformed binary microarchitecture simulated by the phase-field method.
Supplementary Video 4
Selectively peeling off the uniformly structured microscale torpedoes from the electrode surface by slowly immersing it into water.
Supplementary Video 5
The moving behavior of Ag7O8NO3 microscale torpedoes in 2.5 wt.% H2O2 aqueous solutions.
Source data
Source Data Fig. 1
Unprocessed statistical source data for Fig. 1.
Source Data Fig. 4
Unprocessed statistical source data for Fig. 4.
Source Data Fig. 5
Unprocessed statistical source data for Fig. 5.
Source Data Fig. 7
Unprocessed statistical source data for Fig. 7.
Source Data Fig. 8
Unprocessed statistical source data for Fig. 8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, L., Wang, Y., Jin, S. et al. Rational electrochemical design of hierarchical microarchitectures for SERS sensing applications. Nat. Synth 3, 867–877 (2024). https://doi.org/10.1038/s44160-024-00553-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-024-00553-1
This article is cited by
-
Three-dimensional micro- and nanomanufacturing techniques for high-fidelity wearable bioelectronics
Nature Reviews Electrical Engineering (2025)