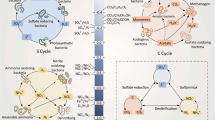
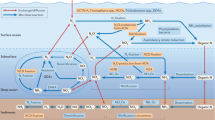
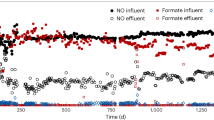
Abstract
Nitrogen-cycling microorganisms play essential roles in biological wastewater treatment, where nitrogen is removed with substantial energy and chemical consumption and greenhouse gas emissions. The discoveries of new nitrogen-cycling microorganisms paved the way for a remarkable paradigm shift from energy-negative and carbon-positive to energy-positive and carbon-neutral wastewater management. This Review reflects on the trajectory of these microbial discoveries and summarizes the technological progress enabled by them thus far. By bridging the gap between environmental microbiologists and water engineers, who are both interested in these new nitrogen-cycling microorganisms but with different focuses and expertise, this Review acknowledges the challenges encountered and illuminates the exciting future ahead. The continued close collaboration between scientists and engineers will keep redefining the landscape of wastewater management.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kuypers, M. M., Marchant, H. K. & Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 16, 263–276 (2018).
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196 (2010).
Fowler, D. et al. The global nitrogen cycle in the twenty-first century. Phil. Trans. R. Soc. B 368, 20130164 (2013).
Munasinghe-Arachchige, S. P. & Nirmalakhandan, N. Nitrogen-fertilizer recovery from the centrate of anaerobically digested sludge. Environ. Sci. Technol. Lett. 7, 450–459 (2020).
Wuhrmann, K. Nitrogen removal in sewage treatment processes: with 9 figures in the text and on 2 folders. Int. Ver. Theor. Angew. Limnol. 15, 580–596 (1964).
Ludzack, F. & Ettinger, M. Controlling operation to minimize activated sludge effluent nitrogen. J. Water Pollut. Control Fed. 34, 920–931 (1962).
Treusch, A. H. et al. Novel genes for nitrite reductase and Amo‐related proteins indicate a role of uncultivated mesophilic crenarchaeota in nitrogen cycling. Environ. Microbiol. 7, 1985–1995 (2005).
Venter, J. C. et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304, 66–74 (2004).
Könneke, M. et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437, 543–546 (2005).
Qin, W. et al. Alternative strategies of nutrient acquisition and energy conservation map to the biogeography of marine ammonia-oxidizing archaea. ISME J. 14, 2595–2609 (2020).
Könneke, M. et al. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc. Natl Acad. Sci. USA 111, 8239–8244 (2014).
Jung, M.-Y. et al. Ammonia-oxidizing archaea possess a wide range of cellular ammonia affinities. ISME J. 16, 272–283 (2022).
Martens-Habbena, W., Berube, P. M., Urakawa, H., José, R. & Stahl, D. A. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461, 976–979 (2009).
Kozlowski, J. A., Stieglmeier, M., Schleper, C., Klotz, M. G. & Stein, L. Y. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. ISME J 10, 1836–1845 (2016).
Carini, P., Dupont, C. L. & Santoro, A. E. Patterns of thaumarchaeal gene expression in culture and diverse marine environments. Environ. Microbiol. 20, 2112–2124 (2018).
Walker, C. B. et al. Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl Acad. Sci. USA 107, 8818–8823 (2010).
Stein, L. Y. et al. Comment on “A critical review on nitrous oxide production by ammonia-oxidizing archaea” by Lan Wu, Xueming Chen, Wei Wei, Yiwen Liu, Dongbo Wang, and Bing-Jie Ni. Environ. Sci. Technol. 55, 797–798 (2020).
Stieglmeier, M. et al. Aerobic nitrous oxide production through N-nitrosating hybrid formation in ammonia-oxidizing archaea. ISME J. 8, 1135–1146 (2014).
Jung, M.-Y. et al. Indications for enzymatic denitrification to N2O at low pH in an ammonia-oxidizing archaeon. ISME J. 13, 2633–2638 (2019).
Wan, X. S. et al. Pathways of N2O production by marine ammonia-oxidizing archaea determined from dual-isotope labeling. Proc. Natl Acad. Sci. USA 120, e2220697120 (2023).
Costa, E., Pérez, J. & Kreft, J.-U. Why is metabolic labour divided in nitrification? Trends Microbiol 14, 213–219 (2006).
Daims, H. et al. Complete nitrification by Nitrospira bacteria. Nature 528, 504–509 (2015).
van Kessel, M. A. et al. Complete nitrification by a single microorganism. Nature 528, 555–559 (2015).
Palomo, A. et al. Comparative genomics sheds light on niche differentiation and the evolutionary history of comammox. Nitrospira. ISME J. 12, 1779–1793 (2018).
Kits, K. D. et al. Low yield and abiotic origin of N2O formed by the complete nitrifier Nitrospira inopinata. Nat. Commun. 10, 1836 (2019).
Kits, K. D. et al. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature 549, 269–272 (2017).
Sakoula, D. et al. Enrichment and physiological characterization of a novel comammox Nitrospira indicates ammonium inhibition of complete nitrification. ISME J. 15, 1010–1024 (2021).
Fumasoli, A., Morgenroth, E. & Udert, K. M. Modeling the low pH limit of Nitrosomonas eutropha in high-strength nitrogen wastewaters. Water Res 83, 161–170 (2015).
Hayatsu, M. et al. An acid-tolerant ammonia-oxidizing γ-proteobacterium from soil. ISME J. 11, 1130–1141 (2017).
Picone, N. et al. Ammonia oxidation at pH 2.5 by a new gammaproteobacterial ammonia-oxidizing bacterium. ISME J. 15, 1150–1164 (2021).
Wang, Z. et al. Robust nitritation sustained by acid-tolerant ammonia-oxidizing bacteria. Environ. Sci. Technol. 55, 2048–2056 (2021).
Li, J. et al. Achieving stable partial nitritation in an acidic nitrifying bioreactor. Environ. Sci. Technol. 54, 456–463 (2019).
Fumasoli, A. et al. Growth of Nitrosococcus-related ammonia oxidizing bacteria coincides with extremely low pH values in wastewater with high ammonia content. Environ. Sci. Technol. 51, 6857–6866 (2017).
Krulwich, T. A., Sachs, G. & Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 9, 330–343 (2011).
Wang, Z. et al. Stoichiometric and kinetic characterization of an acid-tolerant ammonia oxidizer ‘Candidatus Nitrosoglobus’. Water Res 196, 117026 (2021).
Ni, G. et al. Metabolic interactions of a minimal bacterial consortium drive robust nitritation at acidic pH. Preprint at BioRxiv https://doi.org/10.1101/2023.10.29.564480 (2023).
Blum, J. M. et al. The pH dependency of N‐converting enzymatic processes, pathways and microbes: effect on net N2O production. Environ. Microbiol. 20, 1623–1640 (2018).
Su, Q., Domingo-Félez, C., Jensen, M. M. & Smets, B. F. Abiotic nitrous oxide (N2O) production is strongly pH dependent, but contributes little to overall N2O emissions in biological nitrogen removal systems. Environ. Sci. Technol. 53, 3508–3516 (2019).
Kits, K. D., Klotz, M. G. & Stein, L. Y. Methane oxidation coupled to nitrate reduction under hypoxia by the Gammaproteobacterium Methylomonas denitrificans, sp. nov. type strain FJG1. Environ. Microbiol. 17, 3219–3232 (2015).
Poret-Peterson, A. T., Graham, J. E., Gulledge, J. & Klotz, M. G. Transcription of nitrification genes by the methane-oxidizing bacterium, Methylococcus capsulatus strain Bath. ISME J 2, 1213–1220 (2008).
Daebeler, A. et al. Exploring the upper pH limits of nitrite oxidation: diversity, ecophysiology, and adaptive traits of haloalkalitolerant Nitrospira. ISME J. 14, 2967–2979 (2020).
Hankinson, T. & Schmidt, E. An acidophilic and a neutrophilic Nitrobacter strain isolated from the numerically predominant nitrite-oxidizing population of an acid forest soil. Appl. Environ. Microb. 54, 1536–1540 (1988).
Laloo, A. E. Mechanism of Action of Free Nitrous Acid (FNA) on Nitrifiers. PhD Thesis, Univ. Queensland (2019).
Wu, M. R. et al. Novel Alcaligenes ammonioxydans sp. nov. from wastewater treatment sludge oxidizes ammonia to N2 with a previously unknown pathway. Environ. Microbiol. 23, 6965–6980 (2021).
Lenferink, W. B., Bakken, L. R., Jetten, M. S. M., van Kessel, M. A. H. J. & Lücker, S. Hydroxylamine production by Alcaligenes faecalis challenges the paradigm of heterotrophic nitrification. Sci. Adv 10, eadl3587 (2024).
Mulder, A., Vandegraaf, A. A., Robertson, L. A. & Kuenen, J. G. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol. Ecol 16, 177–183 (1995).
Strous, M. et al. Missing lithotroph identified as new planctomycete. Nature 400, 446–449 (1999).
Oshiki, M., Ali, M., Shinyako-Hata, K., Satoh, H. & Okabe, S. Hydroxylamine-dependent anaerobic ammonium oxidation (anammox) by ‘Candidatus Brocadia sinica’. Environ. Microbiol. 18, 3133–3143 (2016).
Lawson, C. E. et al. Metabolic network analysis reveals microbial community interactions in anammox granules. Nat. Commun. 8, 15416 (2017).
Li, J., Liu, T., McIlroy, S. J., Tyson, G. W. & Guo, J. Phylogenetic and metabolic diversity of microbial communities performing anaerobic ammonium and methane oxidations under different nitrogen loadings. ISME Commun 3, 39 (2023).
Kartal, B. et al. How to make a living from anaerobic ammonium oxidation. FEMS Microbiol. Rev. 37, 428–461 (2013).
Dietl, A. et al. The inner workings of the hydrazine synthase multiprotein complex. Nature 527, 394–397 (2015).
Shaw, D. R. et al. Extracellular electron transfer-dependent anaerobic oxidation of ammonium by anammox bacteria. Nat. Commun. 11, 2058 (2020).
Strous, M. et al. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature 440, 790–794 (2006).
Clément, J.-C., Shrestha, J., Ehrenfeld, J. G. & Jaffé, P. R. Ammonium oxidation coupled to dissimilatory reduction of iron under anaerobic conditions in wetland soils. Soil Biol. Biochem. 37, 2323–2328 (2005).
Ding, L.-J., An, X.-L., Li, S., Zhang, G.-L. & Zhu, Y.-G. Nitrogen loss through anaerobic ammonium oxidation coupled to iron reduction from paddy soils in a chronosequence. Environ. Sci. Technol. 48, 10641–10647 (2014).
Yang, W. H., Weber, K. A. & Silver, W. L. Nitrogen loss from soil through anaerobic ammonium oxidation coupled to iron reduction. Nat. Geosci. 5, 538–541 (2012).
Huang, S. & Jaffé, P. R. Isolation and characterization of an ammonium-oxidizing iron reducer: Acidimicrobiaceae sp. A6. PLoS ONE 13, e0194007 (2018).
Jaffé, P. R. et al. Defluorination of PFAS by Acidimicrobium sp. strain A6 and potential applications for remediation. Method Enzymol. 696, 287–320 (2024).
Liu, T., Chen, D., Li, X. & Li, F. Microbially mediated coupling of nitrate reduction and Fe (II) oxidation under anoxic conditions. FEMS Microbiol. Ecol 95, fiz030 (2019).
Zhang, X., Li, A., Szewzyk, U. & Ma, F. Improvement of biological nitrogen removal with nitrate-dependent Fe (II) oxidation bacterium Aquabacterium parvum B6 in an up-flow bioreactor for wastewater treatment. Bioresour. Technol. 219, 624–631 (2016).
Muyzer, G., Kuenen, J. G. & Robertson, L. A. in The Prokaryotes Prokaryotic Physiology and Biochemistry (eds Rosenberg, E. et al.) 555–588 (2013).
Szekeres, S., Kiss, I., Kalman, M. & Soares, M. I. M. Microbial population in a hydrogen-dependent denitrification reactor. Water Res 36, 4088–4094 (2002).
Raghoebarsing, A. A. et al. A microbial consortium couples anaerobic methane oxidation to denitrification. Nature 440, 918–921 (2006).
Ettwig, K. F. et al. Nitrite-driven anaerobic methane oxidation by oxygenic bacteria. Nature 464, 543–548 (2010).
Haroon, M. F. et al. Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage. Nature 500, 567–570 (2013).
Ettwig, K. F. et al. Archaea catalyze iron-dependent anaerobic oxidation of methane. Proc. Natl Acad. Sci. USA 113, 12792–12796 (2016).
Yao, X. et al. Methane-dependent complete denitrification by a single Methylomirabilis bacterium. Nat. Microbiol. 9, 464–476 (2024).
Wu, M. et al. Anaerobic oxidation of propane coupled to nitrate reduction by a lineage within the class Symbiobacteriia. Nat. Commun. 13, 6115 (2022).
Wu, M. et al. Nitrate-driven anaerobic oxidation of ethane and butane by bacteria. ISME J 18, wrad011 (2024).
Garrido-Amador, P. et al. Enrichment and characterization of a nitric oxide-reducing microbial community in a continuous bioreactor. Nat. Microbiol. 8, 1574–1586 (2023).
Conthe, M. et al. Life on N2O: deciphering the ecophysiology of N2O respiring bacterial communities in a continuous culture. ISME J. 12, 1142–1153 (2018).
Yoon, S., Nissen, S., Park, D., Sanford, R. A. & Löffler, F. E. Nitrous oxide reduction kinetics distinguish bacteria harboring clade I nosZ from those harboring clade II nosZ. Appl. Environ. Microb. 82, 3793–3800 (2016).
Jones, C. M., Graf, D. R. H., Bru, D., Philippot, L. & Hallin, S. The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. ISME J 7, 417–426 (2013).
Han, P. et al. N2O and NOy production by the comammox bacterium Nitrospira inopinata in comparison with canonical ammonia oxidizers. Water Res. 190, 116728 (2021).
Conthe, M. et al. Denitrification as an N2O sink. Water Res. 151, 381–387 (2019).
Valk, L. C. et al. Exploring the microbial influence on seasonal nitrous oxide concentration in a full-scale wastewater treatment plant using metagenome assembled genomes. Water Res. 219, 118563 (2022).
Qi, C. et al. Organic carbon determines nitrous oxide consumption activity of clade I and II nosZ bacteria: genomic and biokinetic insights. Water Res. 209, 117910 (2022).
Suenaga, T. et al. Enrichment, isolation, and characterization of high-affinity N2O-reducing bacteria in a gas-permeable membrane reactor. Environ. Sci. Technol. 53, 12101–12112 (2019).
Suenaga, T. et al. Immobilization of Azospira sp. strain I13 by gel entrapment for mitigation of N2O from biological wastewater treatment plants: biokinetic characterization and modeling. J. Biosci. Bioeng. 126, 213–219 (2018).
Liu, T. et al. Simultaneous removal of dissolved methane and nitrogen from synthetic mainstream anaerobic effluent. Environ. Sci. Technol. 54, 7629–7638 (2020).
Liu, T., Hu, S., Yuan, Z. & Guo, J. Simultaneous dissolved methane and nitrogen removal from low-strength wastewater using anaerobic granule-based sequencing batch reactor. Water Res 242, 120194 (2023).
Silva-Teira, A., Sanchez, A., Buntner, D., Rodriguez-Hernandez, L. & Garrido, J. M. Removal of dissolved methane and nitrogen from anaerobically treated effluents at low temperature by MBR post-treatment. Chem. Eng. J. 326, 970–979 (2017).
Chen, X. et al. A new approach to simultaneous ammonium and dissolved methane removal from anaerobic digestion liquor: a model-based investigation of feasibility. Water Res. 85, 295–303 (2015).
Lu, Y. et al. Coupling partial nitritation, anammox and n-DAMO in a membrane aerated biofilm reactor for simultaneous dissolved methane and nitrogen removal. Water Res. 255, 121511 (2024).
Tang, C. J. et al. Performance of high-loaded anammox UASB reactors containing granular sludge. Water Res. 45, 135–144 (2011).
Fan, S.-Q. et al. Granular sludge coupling nitrate/nitrite dependent anaerobic methane oxidation with anammox: from proof-of-concept to high rate nitrogen removal. Environ. Sci. Technol. 54, 297–305 (2019).
Niu, C. et al. Superior mainstream partial nitritation in an acidic membrane-aerated biofilm reactor. Water Res. 257, 121692 (2024).
Cao, Y., van Loosdrecht, M. C. M. & Daigger, G. T. Mainstream partial nitritation–anammox in municipal wastewater treatment: status, bottlenecks, and further studies. Appl. Microbiol. Biotechnol. 101, 1365–1383 (2017).
Wang, Z. et al. Acidic aerobic digestion of anaerobically-digested sludge enabled by a novel ammonia-oxidizing bacterium. Water Res. 194, 116962 (2021).
Zhang, L. et al. Increasing capacity of an anaerobic sludge digester through FNA pre-treatment of thickened waste activated sludge. Water Res. 149, 406–413 (2019).
Hu, Z. et al. Adaptation of anammox process for nitrogen removal from acidic nitritation effluent in a low pH moving bed biofilm reactor. Water Res. 243, 120370 (2023).
Xia, J., Ni, G., Wang, Y., Zheng, M. & Hu, S. Mycolicibacter acidiphilus sp. nov., an extremely acid-tolerant member of the genus Mycolicibacter. Int. J. Syst. Evol. Micr 72, 005419 (2022).
Godfrey, B. et al. Co-immobilization of AOA strains with anammox bacteria in three different synthetic bio-granules maintained under two substrate-level conditions. Chemosphere 342, 140192 (2023).
Li, B. et al. Mainstream nitrogen removal from low temperature and low ammonium strength municipal wastewater using hydrogel-encapsulated comammox and anammox. Water Res. 242, 120303 (2023).
Shao, Y.-H. & Wu, J.-H. Comammox Nitrospira species dominate in an efficient partial nitrification–anammox bioreactor for treating ammonium at low loadings. Environ. Sci. Technol. 55, 2087–2098 (2021).
Lim, Z. K. et al. Versatility of nitrite/nitrate-dependent anaerobic methane oxidation (n-DAMO): first demonstration with real wastewater. Water Res. 194, 116912 (2021).
Deng, Y.-F. et al. Coupling sulfur-based denitrification with anammox for effective and stable nitrogen removal: a review. Water Res. 224, 119051 (2022).
Deng, Y.-F. et al. Exploration and verification of the feasibility of sulfide-driven partial denitrification coupled with anammox for wastewater treatment. Water Res. 193, 116905 (2021).
Feng, F. et al. Quantification of enhanced nitrogen removal pathways of pyrite interaction with anammox sludge system. Chem. Eng. J. 459, 141519 (2023).
Yang, Y., Xiao, C., Lu, J. & Zhang, Y. Fe (III)/Fe (II) forwarding a new anammox-like process to remove high-concentration ammonium using nitrate as terminal electron acceptor. Water Res 172, 115528 (2020).
Liu, W. et al. Temperature-resilient superior performances by coupling partial nitritation/anammox and iron-based denitrification with granular formation. Water Res. 254, 121424 (2024).
Men, Y. et al. Biotransformation of two pharmaceuticals by the ammonia-oxidizing Archaeon Nitrososphaera gargensis. Environ. Sci. Technol. 50, 4682–4692 (2016).
Han, P. et al. Specific micropollutant biotransformation pattern by the comammox bacterium Nitrospira inopinata. Environ. Sci. Technol. 53, 8695–8705 (2019).
Martínez-Quintela, M. et al. Cometabolic removal of organic micropollutants by enriched nitrite-dependent anaerobic methane oxidizing cultures. J. Hazard. Mater. 402, 123450 (2021).
Huang, J. et al. Unraveling pharmaceuticals removal in a sulfur-driven autotrophic denitrification process: performance, kinetics and mechanisms. Chin. Chem. Lett. 34, 107433 (2023).
Cheng, Z. et al. Study of free nitrous acid (FNA)-based elimination of sulfamethoxazole: kinetics, transformation pathways, and toxicity assessment. Water Res. 189, 116629 (2021).
Liu, W. et al. Increasing the removal efficiency of antibiotic resistance through anaerobic digestion with free nitrous acid pretreatment. J. Hazard. Mater. 438, 129535 (2022).
Cai, C. et al. Acetate production from anaerobic oxidation of methane via intracellular storage compounds. Environ. Sci. Technol. 53, 7371–7379 (2019).
Zhang, J. et al. Feasibility of methane bioconversion to methanol by acid-tolerant ammonia-oxidizing bacteria. Water Res. 197, 117077 (2021).
Larsen, T. A., Riechmann, M. E. & Udert, K. M. State of the art of urine treatment technologies: a critical review. Water Res. X 13, 100114 (2021).
Zuo, Z. et al. The advantage of a two-stage nitrification method for fertilizer recovery from human urine. Water Res. 235, 119932 (2023).
Larsen, T. A., Gruendl, H. & Binz, C. The potential contribution of urine source separation to the SDG agenda—a review of the progress so far and future development options. Environ. Sci. Water Res. Technol. 7, 1161–1176 (2021).
Zuo, Z., Zheng, M., Liu, T., Peng, Y. & Yuan, Z. New perspectives in free nitrous acid (FNA) uses for sustainable wastewater management. Front. Environ. Sci. Eng. 18, 26 (2024).
Scherson, Y. D. et al. Nitrogen removal with energy recovery through N2O decomposition. Energy Environ. Sci. 6, 241–248 (2013).
Oshiki, M. et al. Biosynthesis of hydrazine from ammonium and hydroxylamine using an anaerobic ammonium oxidizing bacterium. Biotechnol. Lett. 42, 979–985 (2020).
Straka, L. L., Meinhardt, K. A., Bollmann, A., Stahl, D. A. & Winkler, M.-K. Affinity informs environmental cooperation between ammonia-oxidizing archaea (AOA) and anaerobic ammonia-oxidizing (anammox) bacteria. ISME J 13, 1997–2004 (2019).
Belser, L. & Schmidt, E. Growth and oxidation kinetics of three genera of ammonia oxidizing nitrifiers. FEMS Microbiol. Lett 7, 213–216 (1980).
Boon, B. & Laudelout, H. Kinetics of nitrite oxidation by Nitrobacter winogradskyi. Biochem. J 85, 440 (1962).
Winkler, M. K. et al. Modelling simultaneous anaerobic methane and ammonium removal in a granular sludge reactor. Water Res. 73, 323–331 (2015).
He, L. et al. A methanotrophic bacterium to enable methane removal for climate mitigation. Proc. Natl Acad. Sci. USA 120, e2310046120 (2023).
Zhao, J. et al. Selective enrichment of comammox Nitrospira in a moving bed biofilm reactor with sufficient oxygen supply. Environ. Sci. Technol. 56, 13338–13346 (2022).
Cotto, I. et al. Long solids retention times and attached growth phase favor prevalence of comammox bacteria in nitrogen removal systems. Water Res. 169, 115268 (2020).
Liu, C. et al. Rapid formation of granules coupling n-DAMO and anammox microorganisms to remove nitrogen. Water Res. 194, 116963 (2021).
Liu, T. et al. Temperature-tolerated mainstream nitrogen removal by anammox and nitrite/nitrate-dependent anaerobic methane oxidation in a membrane biofilm reactor. Environ. Sci. Technol. 54, 3012–3021 (2020).
Li, J. et al. Selective enrichment and metagenomic analysis of three novel comammox Nitrospira in a urine-fed membrane bioreactor. ISME Commun. 1, 7 (2021).
Allegue, T., Arias, A., Fernandez-Gonzalez, N., Omil, F. & Garrido, J. M. Enrichment of nitrite-dependent anaerobic methane oxidizing bacteria in a membrane bioreactor. Chem. Eng. J. 347, 721–730 (2018).
Lotti, T., Kleerebezem, R., Abelleira-Pereira, J., Abbas, B. & van Loosdrecht, M. Faster through training: the anammox case. Water Res. 81, 261–268 (2015).
Zhang, L. et al. Maximum specific growth rate of anammox bacteria revisited. Water Res. 116, 296–303 (2017).
Guerrero-Cruz, S. et al. Key physiology of a nitrite-dependent methane-oxidizing enrichment culture. Appl. Environ. Microb. 85, 00124–00119 (2019).
Kartal, B. et al. Anammox bacteria disguised as denitrifiers: nitrate reduction to dinitrogen gas via nitrite and ammonium. Environ. Microbiol. 9, 635–642 (2007).
Guven, D. et al. Propionate oxidation by and methanol inhibition of anaerobic ammonium-oxidizing bacteria. Appl. Environ. Microb. 71, 1066–1071 (2005).
Lawson, C. E. et al. Autotrophic and mixotrophic metabolism of an anammox bacterium revealed by in vivo 13C and 2H metabolic network mapping. ISME J. 15, 673–687 (2021).
Tourna, M. et al. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc. Natl Acad. Sci. USA 108, 8420–8425 (2011).
Xu, S. et al. Survival strategy of comammox bacteria in a wastewater nutrient removal system with sludge fermentation liquid as additional carbon source. Sci. Total Environ. 802, 149862 (2022).
Kim, J.-G. et al. Hydrogen peroxide detoxification is a key mechanism for growth of ammonia-oxidizing archaea. Proc. Natl Acad. Sci. USA 113, 7888–7893 (2016).
Wett, B. et al. Going for mainstream deammonification from bench to full scale for maximized resource efficiency. Water Sci. Technol. 68, 283–289 (2013).
Lackner, S., Welker, S., Gilbert, E. M. & Horn, H. Influence of seasonal temperature fluctuations on two different partial nitritation-anammox reactors treating mainstream municipal wastewater. Water Sci. Technol 72, 1358–1363 (2015).
Jenni, S., Vlaeminck, S. E., Morgenroth, E. & Udert, K. M. Successful application of nitritation/anammox to wastewater with elevated organic carbon to ammonia ratios. Water Res. 49, 316–326 (2014).
Liu, T. et al. Evaluation of mainstream nitrogen removal by simultaneous partial nitrification, anammox and denitrification (SNAD) process in a granule-based reactor. Chem. Eng. J. 327, 973–981 (2017).
Du, R. et al. Partial denitrification providing nitrite: opportunities of extending application for anammox. Environ. Int. 131, 105001 (2019).
Huang, T. et al. Comammox Nitrospira bacteria are dominant ammonia oxidizers in mainstream nitrification bioreactors emended with sponge carriers. Environ. Sci. Technol. 56, 12584–12591 (2022).
Wang, Y. et al. Seasonal prevalence of ammonia-oxidizing archaea in a full-scale municipal wastewater treatment plant treating saline wastewater revealed by a 6-year time-series analysis. Environ. Sci. Technol. 55, 2662–2673 (2021).
Bernhard, A. E. et al. Abundance of ammonia-oxidizing archaea and bacteria along an estuarine salinity gradient in relation to potential nitrification rates. Appl. Environ. Microbiol. 76, 1285–1289 (2010).
Lackner, S. et al. Full-scale partial nitritation/anammox experiences–an application survey. Water Res. 55, 292–303 (2014).
Li, J. et al. Quantify the contribution of anammox for enhanced nitrogen removal through metagenomic analysis and mass balance in an anoxic moving bed biofilm reactor. Water Res. 160, 178–187 (2019).
Héder, M. From NASA to EU: the evolution of the TRL scale in public sector innovation. Innov. J. 22, 1–23 (2017).
Jin, R.-C., Yang, G.-F., Yu, J.-J. & Zheng, P. The inhibition of the anammox process: a review. Chem. Eng. J. 197, 67–79 (2012).
Huang, D.-Q., Fu, J.-J., Li, Z.-Y., Fan, N.-S. & Jin, R.-C. Inhibition of wastewater pollutants on the anammox process: a review. Sci. Total Environ. 803, 150009 (2022).
Zhang, Y. et al. Hot spring distribution and survival mechanisms of thermophilic comammox. Nitrospira. ISME J. 17, 993–1003 (2023).
Vandekerckhove, T. G., Props, R., Carvajal-Arroyo, J. M., Boon, N. & Vlaeminck, S. E. Adaptation and characterization of thermophilic anammox in bioreactors. Water Res. 172, 115462 (2020).
Koch, H. et al. Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus. Nitrospira. Proc. Natl Acad. Sci. USA 112, 11371–11376 (2015).
Palatinszky, M. et al. Cyanate as an energy source for nitrifiers. Nature 524, 105–108 (2015).
Koch, H. et al. Growth of nitrite-oxidizing bacteria by aerobic hydrogen oxidation. Science 345, 1052–1054 (2014).
Stahl, D. A. & de la Torre, J. R. Physiology and diversity of ammonia-oxidizing archaea. Annu. Rev. Microbiol. 66, 83–101 (2012).
Wang, Q. & He, J. Complete nitrogen removal via simultaneous nitrification and denitrification by a novel phosphate accumulating Thauera sp. strain SND5. Water Res. 185, 116300 (2020).
Versantvoort, W. et al. Multiheme hydroxylamine oxidoreductases produce NO during ammonia oxidation in methanotrophs. Proc. Natl Acad. Sci. USA 117, 24459–24463 (2020).
Huang, S. & Jaffé, P. R. Characterization of incubation experiments and development of an enrichment culture capable of ammonium oxidation under iron-reducing conditions. Biogeosciences 12, 769–779 (2015).
Van Den Berg, E. M., Van Dongen, U., Abbas, B. & Van Loosdrecht, M. C. Enrichment of DNRA bacteria in a continuous culture. ISME J 9, 2153–2161 (2015).
Kraft, B. et al. The environmental controls that govern the end product of bacterial nitrate respiration. Science 345, 676–679 (2014).
Vilardi, K. et al. Co-occurrence and cooperation between comammox and anammox bacteria in a full-scale attached growth municipal wastewater treatment process. Environ. Sci. Technol. 57, 5013–5023 (2023).
Gottshall, E. Y. et al. Sustained nitrogen loss in a symbiotic association of Comammox Nitrospira and Anammox bacteria. Water Res. 202, 117426 (2021).
Pjevac, P. et al. AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. Front. Microbiol. 8, 1508 (2017).
Annavajhala, M. K., Kapoor, V., Santo-Domingo, J. & Chandran, K. Comammox functionality identified in diverse engineered biological wastewater treatment systems. Environ. Sci. Tech. Lett. 5, 110–116 (2018).
Roots, P. et al. Comammox Nitrospira are the dominant ammonia oxidizers in a mainstream low dissolved oxygen nitrification reactor. Water Res. 157, 396–405 (2019).
Yang, Y. et al. Activity and metabolic versatility of complete ammonia oxidizers in full-scale wastewater treatment systems. Mbio 11, e03175–03119 (2020).
Chao, Y., Mao, Y., Yu, K. & Zhang, T. Novel nitrifiers and comammox in a full-scale hybrid biofilm and activated sludge reactor revealed by metagenomic approach. Appl. Microbiol. Biot. 100, 8225–8237 (2016).
Zhou, L.-J. et al. Cometabolic biotransformation and microbial-mediated abiotic transformation of sulfonamides by three ammonia oxidizers. Water Res. 159, 444–453 (2019).
Yan, J. et al. Mimicking the oxygen minimum zones: stimulating interaction of aerobic archaeal and anaerobic bacterial ammonia oxidizers in a laboratory‐scale model system. Environ. Microbiol. 14, 3146–3158 (2012).
Landreau, M., Byson, S. J., You, H., Stahl, D. A. & Winkler, M. K. Effective nitrogen removal from ammonium-depleted wastewater by partial nitritation and anammox immobilized in granular and thin layer gel carriers. Water Res. 183, 116078 (2020).
Zhang, T. et al. Occurrence of ammonia-oxidizing Archaea in activated sludges of a laboratory scale reactor and two wastewater treatment plants. J. Appl. Microbiol 107, 970–977 (2009).
Wells, G. F. et al. Ammonia-oxidizing communities in a highly aerated full-scale activated sludge bioreactor: betaproteobacterial dynamics and low relative abundance of Crenarchaea. Environ. Microbiol. 11, 2310–2328 (2009).
Mußmann, M. et al. Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proc. Natl Acad. Sci. USA 108, 16771–16776 (2011).
Sauder, L. A., Peterse, F., Schouten, S. & Neufeld, J. D. Low-ammonia niche of ammonia-oxidizing archaea in rotating biological contactors of a municipal wastewater treatment plant. Environ. Microbiol. 14, 2589–2600 (2012).
DeLong, E. F. Archaea in coastal marine environments. Proc. Natl Acad. Sci. USA 89, 5685–5689 (1992).
Fuhrman, J. A., McCallum, K. & Davis, A. A. Novel major archaebacterial group from marine plankton. Nature 356, 148–149 (1992).
Faust, V. et al. Ammonia oxidation by novel “Candidatus Nitrosacidococcus urinae” is sensitive to process disturbances at low pH and to iron limitation at neutral pH. Water Res. X 17, 100157 (2022).
Hu, Z., Liu, T., Wang, Z., Meng, J. & Zheng, M. Toward energy neutrality: novel wastewater treatment incorporating acidophilic ammonia oxidation. Environ. Sci. Technol. 57, 4522–4532 (2023).
Fumasoli, A., Etter, B., Sterkele, B., Morgenroth, E. & Udert, K. M. Operating a pilot-scale nitrification/distillation plant for complete nutrient recovery from urine. Water Sci. Technol 73, 215–222 (2016).
Hayatsu, M. The lowest limit of pH for nitrification in tea soil and isolation of an acidophilic ammonia oxidizing bacterium. Soil Sci. Plant Nutr. 39, 219–226 (1993).
Liu, T., Hu, S., Yuan, Z. & Guo, J. High-level nitrogen removal by simultaneous partial nitritation, anammox and nitrite/nitrate-dependent anaerobic methane oxidation. Water Res. 166, 115057 (2019).
Shi, Y. et al. Nitrogen removal from wastewater by coupling anammox and methane-dependent denitrification in a membrane biofilm reactor. Environ. Sci. Technol. 47, 11577–11583 (2013).
Islas-Lima, S., Thalasso, F. & Gomez-Hernandez, J. Evidence of anoxic methane oxidation coupled to denitrification. Water Res. 38, 13–16 (2004).
Mason, I. Methane as a carbon source in biological denitrification. J. Water Pollut. Control Fed. 49, 855–857 (1977).
Thalasso, F., Vallecillo, A., GarciaEncina, P. & FdzPolanco, F. The use of methane as a sole carbon source for wastewater denitrification. Water Res. 31, 55–60 (1997).
Eisentraeger, A., Klag, P., Vansbotter, B., Heymann, E. & Dott, W. Denitrification of groundwater with methane as sole hydrogen donor. Water Res. 35, 2261–2267 (2001).
Le, T. et al. Impact of carbon source and COD/N on the concurrent operation of partial denitrification and anammox. Water Environ. Res. 91, 185–197 (2019).
Cao, Y. et al. The mainstream autotrophic nitrogen removal in the largest full scale activated sludge process in Singapore: process analysis. WEF/IWA Nutrient Removal and Recovery 2013: Trends in Resource Recovery and Use 28–31 (2013).
Third, K. A., Sliekers, A. O., Kuenen, J. G. & Jetten, M. S. M. The CANON system (completely autotrophic nitrogen-removal over nitrite) under ammonium limitation: Interaction and competition between three groups of bacteria. Syst. Appl. Microbiol. 24, 588–596 (2001).
van Dongen, U., Jetten, M. S. & van Loosdrecht, M. C. The SHARON-Anammox process for treatment of ammonium rich wastewater. Water Sci. Technol. 44, 153–160 (2001).
Broda, E. Two kinds of lithotrophs missing in nature. Z. Allg. Mikrobiol. 17, 491–493 (1977).
Winogradsky, S. Recherches sur les organisms de la nitrification. Ann. Inst. Pasteur (Paris) 4, 213–231 (1890).
Gayon, U. & Dupetit, G. Recherches sur la reduction des nitrates par les infiniment petits. Mem. Soc. Sci. Phys. Nat. Bord. 3, 201–307 (1886).
Larsen, T., Udert, K. & Lienert, J. Source Separation and Decentralization for Wastewater Management (IWA Publishing, 2013).
Galloway, J. N. et al. The nitrogen cascade. Bioscience 53, 341–356 (2003).
Sigurdarson, J. J., Svane, S. & Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol 17, 241–258 (2018).
Qin, W. et al. Ammonia-oxidizing bacteria and archaea exhibit differential nitrogen source preferences. Nat. Microbiol. 9, 524–536 (2024).
Daims, H., Lücker, S. & Wagner, M. A new perspective on microbes formerly known as nitrite-oxidizing bacteria. Trends Microbiol 24, 699–712 (2016).
Su, Z., Liu, T., Guo, J. & Zheng, M. Nitrite oxidation in wastewater treatment: microbial adaptation and suppression challenges. Environ. Sci. Technol. 57, 12557–12570 (2023).
IPCC Climate Change 2021: The Physical Science Basis (eds et al.) (Cambridge Univ. Press, 2023).
Tian, H. et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586, 248–256 (2020).
Höglund-Isaksson, L., Gómez-Sanabria, A., Klimont, Z., Rafaj, P. & Schöpp, W. Technical potentials and costs for reducing global anthropogenic methane emissions in the 2050 timeframe—results from the GAINS model. Environ. Res. Commun. 2, 025004 (2020).
Water Utilities Unite to Cut Emissions in Race to Zero (Water Sevices Association of Australia, 2021); https://wsaa.stage.wsaa.asn.au/media/water-utilities-unite-cut-emissions-race-zero
Kitamori, K., Manders, T., Dellink, R. & Tabeau, A. OECD Environmental Outlook to 2050: The Consequences of Inaction Report no. 9264122168 (OECD, 2012).
Molinos-Senante, M., Hernández-Sancho, F. & Sala-Garrido, R. Economic feasibility study for wastewater treatment: a cost–benefit analysis. Sci. Total Environ. 408, 4396–4402 (2010).
Beckinghausen, A., Odlare, M., Thorin, E. & Schwede, S. From removal to recovery: an evaluation of nitrogen recovery techniques from wastewater. Appl. Energy 263, 114616 (2020).
Acknowledgements
This work is supported by the Australian Research Council Linkage Project (LP220200963) and Discovery Project (DP230101340). T.L. is a recipient of the Australian Research Council DECRA Fellowship (DE220101310) and Hong Kong Research Grants Council’s Early Career Scheme (PolyU 25238324). H.D. and M.Z. are the recipients of the Australian Research Council Industry Fellowship (IE230100422, IE230100245). H.D. acknowledges funding from the Comammox Research Platform of the University of Vienna and the Austrian Science Fund, Cluster of Excellence COE7. S.L. acknowledges funding from the Dutch Research Council (NWO) grant 016.Vidi.189.050. Z.Y. is a Global STEM Professor jointly funded by the Innovation, Technology and Industry Bureau and Education Bureau of the Government of the Hong Kong Special Administrative Region and acknowledges financial support from the Hong Kong Jockey Club for the JC STEM Lab of Sustainable Urban Water Management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Dario Rangel Shaw and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, T., Duan, H., Lücker, S. et al. Sustainable wastewater management through nitrogen-cycling microorganisms. Nat Water 2, 936–952 (2024). https://doi.org/10.1038/s44221-024-00307-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-024-00307-5
This article is cited by
-
Biohybrid-based pyroelectric bio-denitrification driven by temperature fluctuations
Nature Communications (2025)