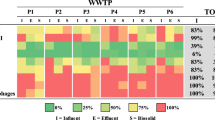
Abstract
The risk posed by microorganisms in diverse environments has emerged as a notable concern. However, existing microbial risk assessment frameworks often lack breadth and coherence. Here, to address this constraint, we developed a cellular spike-in (including one Gram-positive bacterium (G+) and one Gram-negative bacterium (G−)) method that enables absolute quantification of microorganisms in multiple environmental compartments (for example, wastewater, river water and marine water). This method was thoroughly evaluated for consistency, accuracy, feasibility and applicability. Furthermore, we investigated potential biases that might arise from DNA extraction to sequencing under different cell lysis conditions and, importantly, demonstrated that this spike-in absolute quantification method could correct such biases. We then applied this method to various samples to determine the absolute abundance (concentration) of microorganisms, pathogens and antibiotic resistance genes. On the basis of the results, we evaluated the removal efficiencies in terms of pathogens and antibiotic resistance genes in five wastewater treatment plants with different operational modes (for example, chemically enhanced primary treatment, secondary treatment, tertiary treatment and membrane bioreactor). Finally, we developed a risk assessment framework that simplifies complex absolute quantification data into accessible scores, enabling a comprehensive microbial risk evaluation and comparison across diverse environments. This analytical workflow could facilitate informed policymaking and decision-making by authorities based on risk assessment levels, advancing efforts to safeguard public health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the raw sequencing data generated in this study have been deposited in the National Center for Biotechnology Informatio Sequence Read Archive database under BioProject ID: PRJNA1158533.
References
Essack, S. Y. Environment: the neglected component of the One Health triad. Lancet Planet. Health 2, e238–e239 (2018).
Federigi, I. et al. The application of quantitative microbial risk assessment to natural recreational waters: a review. Mar. Pollut. Bull. 144, 334–350 (2019).
Schoen, M. E. et al. Quantitative microbial risk assessment of antimicrobial resistant and susceptible Staphylococcus aureus in reclaimed wastewaters. Environ. Sci. Technol. 55, 15246–15255 (2021).
Astles, K. L. et al. An ecological method for qualitative risk assessment and its use in the management of fisheries in New South Wales, Australia. Fish. Res. 82, 290–303 (2006).
Hordyk, A. R. & Carruthers, T. R. A quantitative evaluation of a qualitative risk assessment framework: examining the assumptions and predictions of the productivity susceptibility analysis (PSA). PLoS ONE 13, e0198298 (2018).
Lim, K.-Y. et al. Evaluation of the dry and wet weather recreational health risks in a semi-enclosed marine embayment in Southern California. Water Res. 111, 318–329 (2017).
Tymensen, L. D. et al. Comparative accessory gene fingerprinting of surface water Escherichia coli reveals genetically diverse naturalized population. J. Appl. Microbiol. 119, 263–277 (2015).
Mbanga, J. et al. Quantitative microbial risk assessment for waterborne pathogens in a wastewater treatment plant and its receiving surface water body. BMC Microbiol. 20, 346 (2020).
Owens, C. E. et al. Implementation of quantitative microbial risk assessment (QMRA) for public drinking water supplies: systematic review. Water Res. 174, 115614 (2020).
Miliotis, M. et al. Role of epidemiology in microbial risk assessment. Food Addit. Contam. 25, 1052–1057 (2008).
Goh, S. G. et al. A new modelling framework for assessing the relative burden of antimicrobial resistance in aquatic environments. J. Hazard. Mater. 424, 127621 (2022).
Shao, Y. et al. A systematic review on antibiotics misuse in livestock and aquaculture and regulation implications in China. Sci. Total Environ. 798, 149205 (2021).
Shao, S. et al. Research progress on distribution, migration, transformation of antibiotics and antibiotic resistance genes (ARGs) in aquatic environment. Crit. Rev. Biotechnol. 38, 1195–1208 (2018).
Huemer, M. et al. Antibiotic resistance and persistence—implications for human health and treatment perspectives. EMBO Rep. 21, e51034 (2020).
Martínez, J. L. Ecology and evolution of chromosomal gene transfer between environmental microorganisms and pathogens. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.mtbp-0006-2016 (2018).
Che, Y. et al. Mobile antibiotic resistome in wastewater treatment plants revealed by Nanopore metagenomic sequencing. Microbiome 7, 44 (2019).
Forster, S. C. et al. Strain-level characterization of broad host range mobile genetic elements transferring antibiotic resistance from the human microbiome. Nat. Commun. 13, 1445 (2022).
Larsson, D. & Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 20, 257–269 (2022).
Zhang, A.-N. et al. An omics-based framework for assessing the health risk of antimicrobial resistance genes. Nat. Commun. 12, 4765 (2021).
Oliveira, D. M. P. D. et al. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. https://doi.org/10.1128/cmr.00181-19 (2020).
Zhen, X. et al. Economic burden of antibiotic resistance in ESKAPE organisms: a systematic review. Antimicrob. Resist. Infect. Control 8, 137 (2019).
Reyneke, B. et al. Comparison of EMA-, PMA- and DNase qPCR for the determination of microbial cell viability. Appl. Microbiol. Biotechnol. 101, 7371–7383 (2017).
McLain, J. E. et al. Culture‐based methods for detection of antibiotic resistance in agroecosystems: advantages, challenges, and gaps in knowledge. J. Environ. Qual. 45, 432–440 (2016).
Ko, K. K., Chng, K. R. & Nagarajan, N. Metagenomics-enabled microbial surveillance. Nat. Microbiol. 7, 486–496 (2022).
Xu, H.-S. et al. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb. Ecol. 8, 313–323 (1982).
Frossard, A., Hammes, F. & Gessner, M. O. Flow cytometric assessment of bacterial abundance in soils, sediments and sludge. Front. Microbiol. 7, 195298 (2016).
Ruijter, J. et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 37, e45 (2009).
Krehenwinkel, H. et al. Estimating and mitigating amplification bias in qualitative and quantitative arthropod metabarcoding. Sci. Rep. 7, 17668 (2017).
Vandeputte, D. et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551, 507–511 (2017).
Zhang, Z. et al. Soil bacterial quantification approaches coupling with relative abundances reflecting the changes of taxa. Sci. Rep. 7, 4837 (2017).
Ji, B. W. et al. Quantifying spatiotemporal variability and noise in absolute microbiota abundances using replicate sampling. Nat. Methods 16, 731–736 (2019).
Wang, C. et al. Absolute quantification and genome-centric analyses elucidate the dynamics of microbial populations in anaerobic digesters. Water Res. 224, 119049 (2022).
Tkacz, A., Hortala, M. & Poole, P. S. Absolute quantitation of microbiota abundance in environmental samples. Microbiome 6, 110 (2018).
Yang, Y. et al. Rapid absolute quantification of pathogens and ARGs by nanopore sequencing. Sci. Total Environ. 809, 152190 (2022).
Yang, Y. et al. QMRA of beach water by Nanopore sequencing-based viability-metagenomics absolute quantification. Water Res. 235, 119858 (2023).
Smets, W. et al. A method for simultaneous measurement of soil bacterial abundances and community composition via 16S rRNA gene sequencing. Soil Biol. Biochem. 96, 145–151 (2016).
Crossette, E. et al. Metagenomic quantification of genes with internal standards. mBio https://doi.org/10.1128/mbio.03173-20 (2021).
Janda, J. M. & Abbott, S. L. The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 23, 35–73 (2010).
Wong, D. et al. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin. Microbiol. Rev. 30, 409–447 (2017).
Wexler, A. G. & Goodman, A. L. An insider’s perspective: Bacteroides as a window into the microbiome. Nat. Microbiol. 2, 17026 (2017).
Radomski, N. et al. Mycobacterium behavior in wastewater treatment plant, a bacterial model distinct from Escherichia coli and Enterococci. Environ. Sci. Technol. 45, 5380–5386 (2011).
Pendleton, J. N., Gorman, S. P. & Gilmore, B. F. Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 11, 297–308 (2013).
Zhang, S. et al. Dissemination of antibiotic resistance genes (ARGs) via integrons in Escherichia coli: a risk to human health. Environ. Pollut. 266, 115260 (2020).
Hirshfeld, B. et al. Prevalence and antimicrobial resistance profiles of Vibrio spp. and Enterococcus spp. in retail shrimp in Northern California. Front. Microbiol. 14, 1192769 (2023).
Martinez, J. L. et al. Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol. Rev. 33, 430–449 (2009).
Deamer, D., Akeson, M. & Branton, D. Three decades of nanopore sequencing. Nat. Biotechnol. 34, 518–524 (2016).
Bayley, H. Nanopore sequencing: from imagination to reality. Clin. Chem. 61, 25–31 (2015).
Lin, B., Hui, J. & Mao, H. Nanopore technology and its applications in gene sequencing. Biosensors 11, 214 (2021).
Contijoch, E. J. et al. Gut microbiota density influences host physiology and is shaped by host and microbial factors. eLife 8, e40553 (2019).
Yin, X. et al. Toward a universal unit for quantification of antibiotic resistance genes in environmental samples. Environ. Sci. Technol. 57, 9713–9721 (2023).
Yin, X. et al. An assessment of resistome and mobilome in wastewater treatment plants through temporal and spatial metagenomic analysis. Water Res. 209, 117885 (2022).
Offenbaume, K. L., Bertone, E. & Stewart, R. A. Monitoring approaches for faecal indicator bacteria in water: visioning a remote real-time sensor for E. coli and Enterococci. Water 12, 2591 (2020).
Castañeda-Barba, S., Top, E. M. & Stalder, T. Plasmids, a molecular cornerstone of antimicrobial resistance in the One Health era. Nat. Rev. Microbiol. 22, 18–32 (2024).
Ellabaan, M. M. et al. Forecasting the dissemination of antibiotic resistance genes across bacterial genomes. Nat. Commun. 12, 2435 (2021).
Priti, K. & Kumar, P. A critical evaluation of air quality index models (1960–2021). Environ. Monit. Assess. 194, 324 (2022).
Child, H. T. et al. Comparative evaluation of soil DNA extraction kits for long read metagenomic sequencing. Access Microbiol. 6, 000868.v3 (2024).
Seethalakshmi, P. et al. Comparative analysis of commercially available kits for optimal DNA extraction from bovine fecal samples. Arch. Microbiol. 206, 314 (2024).
De Coster, W. et al. NanoPack: visualizing and processing long-read sequencing data. Bioinformatics 34, 2666–2669 (2018).
Wood, D. E., Lu, J. & Langmead, B. Improved metagenomic analysis with Kraken 2. Genome Biol. 20, 257 (2019).
Parks, D. H. et al. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 50, D785–D794 (2022).
Buchfink, B., Reuter, K. & Drost, H.-G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 18, 366–368 (2021).
Buchfink, B., Xie, C. & Huson, D. H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60 (2015).
Yin, X. et al. ARGs-OAP v3.0: antibiotic-resistance gene database curation and analysis pipeline optimization. Engineering 27, 234–241 (2023).
Krawczyk, P. S., Lipinski, L. & Dziembowski, A. PlasFlow: predicting plasmid sequences in metagenomic data using genome signatures. Nucleic Acids Res. 46, e35 (2018).
Pärnänen, K. et al. Maternal gut and breast milk microbiota affect infant gut antibiotic resistome and mobile genetic elements. Nat. Commun. 9, 3891 (2018).
Li, H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics 34, 3094–3100 (2018).
Li, H. New strategies to improve minimap2 alignment accuracy. Bioinformatics 37, 4572–4574 (2021).
Acknowledgements
We thank the financial support of the Theme-based Research Scheme of the Research Grant Council of Hong Kong (grant no. T21-705/20-N) and the Shenzhen Science and Technology Innovation Bureau (no. SGDX20230821091559021). X.S., Y.Y., X.C., J.D. and S.L. thank the University of Hong Kong for their postgraduate studentship. C.W., X.X. and X.M. thank the University of Hong Kong for their postdoctoral fellowship. We also thank the Hong Kong Agriculture, Fisheries and Conservation Department and Hong Kong Environmental Protection Department for sample collection. The computations were performed using research computing facilities offered by Information Technology Services at the University of Hong Kong. We also thank the laboratory technician, V. Fung, for assisting with the experimental process.
Author information
Authors and Affiliations
Contributions
X.S.: conceptualization, methodology, formal analysis, visualization, writing—original draft and investigation. Y.Y.: methodology, writing—review and editing. C.W.: methodology, writing—review and editing. X.X.: methodology, writing—review and editing. X.M.: methodology, writing—review and editing. X.C.: methodology, writing—review and editing. J.D.: methodology, writing—review and editing. S.L.: methodology, writing—review and editing. T.Z.: supervision, resources, conceptualization, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Karina Yew-Hoong Gin and Francis Hassard for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–6 and Figs. 1–4, and References.
Supplementary Tables
Supplementary Tables 1–16.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, X., Yang, Y., Wang, C. et al. Microbial risk assessment across multiple environments based on metagenomic absolute quantification with cellular internal standards. Nat Water 3, 473–485 (2025). https://doi.org/10.1038/s44221-025-00421-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44221-025-00421-y