Abstract
Mammalian cells ubiquitously release membrane-enclosed vesicles, known as extracellular vesicles. These particles carry a variety of molecules that reflect the status of their cells of origin, making them valuable sources for biomarker discovery. Furthermore, extracellular vesicles deliver their cargo locally and systemically to regulate biological processes, piquing interest in modulating extracellular vesicle biogenesis and developing extracellular vesicle-based therapies. Therefore, a thorough understanding of the extracellular vesicle life cycle, from biogenesis and trafficking to degradation, is essential for unlocking their full potential in biomarker identification and for the design of extracellular vesicle-based therapies. In this Review, we start by outlining the key steps in the extracellular vesicle life cycle and highlight remaining open questions. We then discuss the design and application of genetically encoded systems that can be applied to study extracellular vesicle biogenesis and fate. Finally, we highlight technical challenges that remain to be addressed in the engineering and application of genetically encoded systems to extracellular vesicle research.
Key points
-
Extracellular vesicles are shed by cells and implicated in biological processes through diverse mechanisms.
-
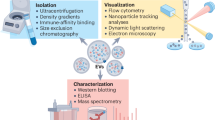
Specific and sensitive characterization of extracellular vesicles is fundamental for identifying extracellular vesicle-based biomarkers and developing therapies. However, their small size and close resemblance to other non-vesicular extracellular particles make their characterization challenging.
-
Genetically encoded systems allow the high-specificity and high-sensitivity characterization of the extracellular vesicle life cycle, from biogenesis and trafficking to degradation.
-
Genetic circuits can be incorporated for the long-term tracking of extracellular vesicle biogenesis and biodistribution in vivo.
-
Caution must be applied when extrapolating knowledge of genetically engineered extracellular vesicles to their native counterparts, which requires complementary approaches.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicles 13, e12404 (2024).
Poupardin, R., Wolf, M. & Strunk, D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv. Drug Deliv. Rev. 176, 113872 (2021).
Lian, M. Q. et al. Plant-derived extracellular vesicles: recent advancements and current challenges on their use for biomedical applications. J. Extracell. Vesicles 11, e12283 (2022).
Hosseini-Giv, N. et al. Bacterial extracellular vesicles and their novel therapeutic applications in health and cancer. Front. Cell. Infect. Microbiol. 12, 962216 (2022).
Rodrigues, M. L. & Nimrichter, L. From fundamental biology to the search for innovation: the story of fungal extracellular vesicles. Eur. J. Cell Biol. 101, 151205 (2022).
Cheng, L. & Hill, A. F. Therapeutically harnessing extracellular vesicles. Nat. Rev. Drug Discov. 21, 379–399 (2022).
Herrmann, I. K., Wood, M. J. A. & Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759 (2021).
Urabe, F. et al. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Physiol. Cell Physiol. 318, C29–C39 (2020).
Shaba, E. et al. Multi-omics integrative approach of extracellular vesicles: a future challenging milestone. Proteomes 10, 12 (2022).
Hendrix, A. et al. Extracellular vesicle analysis. Nat. Rev. Methods Primers 3, 56 (2023).
Rupert, D. L. M., Claudio, V., Lässer, C. & Bally, M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim. Biophys. Acta Gen. Subj. 1861, 3164–3179 (2017).
Hartjes, T., Mytnyk, S., Jenster, G., van Steijn, V. & van Royen, M. E. Extracellular vesicle quantification and characterization: common methods and emerging approaches. Bioengineering 6, 7 (2019).
Penders, J. et al. Single particle automated Raman trapping analysis. Nat. Commun. 9, 4256 (2018).
Ter-Ovanesyan, D. et al. Improved isolation of extracellular vesicles by removal of both free proteins and lipoproteins. eLife 12, e86394 (2023).
Karimi, N. et al. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell. Mol. Life Sci. 75, 2873–2886 (2018).
Wang, T. & Turko, I. V. Proteomic toolbox to standardize the separation of extracellular vesicles and lipoprotein particles. J. Proteome Res. 17, 3104–3113 (2018).
Serrano-Pertierra, E. et al. Extracellular vesicles: current analytical techniques for detection and quantification. Biomolecules 10, 824 (2020).
Verweij, F. J. et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat. Methods 18, 1013–1026 (2021).
Lai, C. P. et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8, 483–494 (2014).
Cheng, A. A. & Lu, T. K. Synthetic biology: an emerging engineering discipline. Annu. Rev. Biomed. Eng. 14, 155–178 (2012).
Kelwick, R. J. R., Webb, A. J., Heliot, A., Segura, C. T. & Freemont, P. S. Opportunities to accelerate extracellular vesicle research with cell-free synthetic biology. J. Extracell. Biol. 2, e90 (2023).
Buzas, E. I. Opportunities and challenges in studying the extracellular vesicle corona. Nat. Cell Biol. 24, 1322–1325 (2022).
van Niel, G., D'Angelo, G. & Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 (2018).
Edgar, J. R., Eden, E. R. & Futter, C. E. Hrs- and CD63-dependent competing mechanisms make different sized endosomal intraluminal vesicles. Traffic 15, 197–211 (2014).
Stuffers, S., Sem Wegner, C., Stenmark, H. & Brech, A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10, 925–937 (2009).
Verweij, F. J. et al. ER membrane contact sites support endosomal small GTPase conversion for exosome secretion. J. Cell Biol. 221, e202112032 (2022).
Miranda, A. M. et al. Neuronal lysosomal dysfunction releases exosomes harboring APP C-terminal fragments and unique lipid signatures. Nat. Commun. 9, 291 (2018).
Eitan, E., Suire, C., Zhang, S. & Mattson, M. P. Impact of lysosome status on extracellular vesicle content and release. Ageing Res. Rev. 32, 65–74 (2016).
Strauss, K. et al. Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann–Pick type C disease. J. Biol. Chem. 285, 26279–26288 (2010).
Teng, F. & Fussenegger, M. Shedding light on extracellular vesicle biogenesis and bioengineering. Adv. Sci. 8, 2003505 (2021).
Margolis, L. & Sadovsky, Y. The biology of extracellular vesicles: the known unknowns. PLoS Biol. 17, e3000363 (2019).
Hurwitz, S. N., Conlon, M. M., Rider, M. A., Brownstein, N. C. & Meckes, D. G. Jr Nanoparticle analysis sheds budding insights into genetic drivers of extracellular vesicle biogenesis. J. Extracell. Vesicles 5, 31295 (2016).
Bost, J. P. et al. Growth media conditions influence the secretion route and release levels of engineered extracellular vesicles. Adv. Healthc. Mater. 11, e2101658 (2021).
Shpigelman, J. et al. Generation and application of a reporter cell line for the quantitative screen of extracellular vesicle release. Front. Pharmacol. 12, 668609 (2021).
Yokoi, A. et al. Mechanisms of nuclear content loading to exosomes. Sci. Adv. 5, eaax8849 (2019).
Dixson, A. C., Dawson, T. R., Di Vizio, D. & Weaver, A. M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 24, 454–476 (2023).
Lenzini, S., Bargi, R., Chung, G. & Shin, J. W. Matrix mechanics and water permeation regulate extracellular vesicle transport. Nat. Nanotechnol. 15, 217–223 (2020).
Gupta, D., Wiklander, O. P. B., Wood, M. J. A. & El-Andaloussi, S. Biodistribution of therapeutic extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids 4, 170–190 (2023).
Sariano, P. A. et al. Convection and extracellular matrix binding control interstitial transport of extracellular vesicles. J. Extracell. Vesicles 12, e12323 (2023).
Debnath, K., Las Heras, K., Rivera, A., Lenzini, S. & Shin, J.-W. Extracellular vesicle–matrix interactions. Nat. Rev. Mater. 8, 390–402 (2023).
Hallal, S., Tűzesi, Á., Grau, G. E., Buckland, M. E. & Alexander, K. L. Understanding the extracellular vesicle surface for clinical molecular biology. J. Extracell. Vesicles 11, e12260 (2022).
Tóth, E. Á. et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J. Extracell. Vesicles 10, e12140 (2021).
Ghosh, P., Liu, Q.-R., Chen, Q., Zhu, M. & Egan, J. M. Pancreatic β cell derived extracellular vesicles containing surface preproinsulin are involved in glucose stimulated insulin secretion. Life Sci. 340, 122460 (2024).
Gupta, D. et al. Quantification of extracellular vesicles in vitro and in vivo using sensitive bioluminescence imaging. J. Extracell. Vesicles 9, 1800222 (2020).
Vidal, M. Exosomes: revisiting their role as “garbage bags”. Traffic 20, 815–828 (2019).
Keller, M. D. et al. Decoy exosomes provide protection against bacterial toxins. Nature 579, 260–264 (2020).
Buzás, E. I., Tóth, E. Á., Sódar, B. W. & Szabó-Taylor, K. É. Molecular interactions at the surface of extracellular vesicles. Semin. Immunopathol. 40, 453–464 (2018).
Roy, S., Hochberg, F. H. & Jones, P. S. Extracellular vesicles: the growth as diagnostics and therapeutics; a survey. J. Extracell. Vesicles 7, 1438720 (2018).
Edelmann, M. J. & Kima, P. E. Current understanding of extracellular vesicle homing/tropism. Zoonoses 2, 14 (2022).
Liam-Or, R. et al. Cellular uptake and in vivo distribution of mesenchymal-stem-cell-derived extracellular vesicles are protein corona dependent. Nat. Nanotechnol. 19, 846–855 (2024).
Heidarzadeh, M., Zarebkohan, A., Rahbarghazi, R. & Sokullu, E. Protein corona and exosomes: new challenges and prospects. Cell Commun. Signal. 21, 64 (2023).
Nishida-Aoki, N., Tominaga, N., Kosaka, N. & Ochiya, T. Altered biodistribution of deglycosylated extracellular vesicles through enhanced cellular uptake. J. Extracell. Vesicles 9, 1713527 (2020).
Gonda, A., Kabagwira, J., Senthil, G. N. & Wall, N. R. Internalization of exosomes through receptor-mediated endocytosis. Mol. Cancer Res. 17, 337–347 (2019).
Qian, F. et al. Analysis and biomedical applications of functional cargo in extracellular vesicles. ACS Nano 16, 19980–20001 (2022).
Bonsergent, E. et al. Quantitative characterization of extracellular vesicle uptake and content delivery within mammalian cells. Nat. Commun. 12, 1864 (2021).
Somiya, M. & Kuroda, S. Real-time luminescence assay for cytoplasmic cargo delivery of extracellular vesicles. Anal. Chem. 93, 5612–5620 (2021).
Vargas, A. et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia. FASEB J. 28, 3703–3719 (2014).
Zhang, X. et al. Programmable extracellular vesicles for macromolecule delivery and genome modifications. Dev. Cell 55, 784–801.e9 (2020).
Somiya, M. & Kuroda, S. Reporter gene assay for membrane fusion of extracellular vesicles. J. Extracell. Vesicles 10, e12171 (2021).
Nambiar, D., Le, Q.-T. & Pucci, F. A case for the study of native extracellular vesicles. Front. Oncol. 14, 1430971 (2024).
Kowal, J. et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl Acad. Sci. USA 113, E968–E977 (2016).
Mathieu, M. et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 12, 4389 (2021).
Liu, D.-A. et al. A phosphoinositide switch mediates exocyst recruitment to multivesicular endosomes for exosome secretion. Nat. Commun. 14, 6883 (2023).
Bebelman, M. P. et al. Real-time imaging of multivesicular body–plasma membrane fusion to quantify exosome release from single cells. Nat. Protoc. 15, 102–121 (2020).
Sung, B. H. et al. A live cell reporter of exosome secretion and uptake reveals pathfinding behavior of migrating cells. Nat. Commun. 11, 2092 (2020).
Verweij, F. J. et al. Quantifying exosome secretion from single cells reveals a modulatory role for GPCR signaling. J. Cell Biol. 217, 1129–1142 (2018).
Morimoto, Y. V., Kojima, S., Namba, K. & Minamino, T. M153R mutation in a pH-sensitive green fluorescent protein stabilizes its fusion proteins. PLoS ONE 6, e19598 (2011).
Liu, A. et al. pHmScarlet is a pH-sensitive red fluorescent protein to monitor exocytosis docking and fusion steps. Nat. Commun. 12, 1413 (2021).
Bebelman, M. P. et al. Exosomal release of the virus-encoded chemokine receptor US28 contributes to chemokine scavenging. iScience 26, 107412 (2023).
Beer, K. B. et al. Degron-tagged reporters probe membrane topology and enable the specific labelling of membrane-wrapped structures. Nat. Commun. 10, 3490 (2019).
Datta, A. et al. High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci. Rep. 8, 8161 (2018).
Ruan, Z. et al. Functional genome-wide short hairpin RNA library screening identifies key molecules for extracellular vesicle secretion from microglia. Cell Rep. 39, 110791 (2022).
Welsh, J. A. et al. A compendium of single extracellular vesicle flow cytometry. J. Extracell. Vesicles 12, e12299 (2023).
Corso, G. et al. Systematic characterization of extracellular vesicles sorting domains and quantification at the single molecule–single vesicle level by fluorescence correlation spectroscopy and single particle imaging. J. Extracell. Vesicles 8, 1663043 (2019).
Bebelman, M. P. et al. Luminescence-based screening for extracellular vesicle release modulators reveals a role for PI4KIIIβ in exosome biogenesis upon lysosome inhibition. Preprint at bioRxiv https://doi.org/10.1101/2023.02.23.529257 (2023).
Chiao, J. J. C., Roberts, J. P., Illner, H. P. & Shires, G. T. Permeability of red-cell membrane to adenosine triphosphate (ATP) molecules during hemorrhagic shock. Surgery 102, 528–533 (1987).
Zheng, W. et al. Identification of scaffold proteins for improved endogenous engineering of extracellular vesicles. Nat. Commun. 14, 4734 (2023).
Dixon, A. S. et al. NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol. 11, 400–408 (2016).
Leidal, A. M. et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat. Cell Biol. 22, 187–199 (2020).
Lu, A. et al. Genome-wide interrogation of extracellular vesicle biology using barcoded miRNAs. eLife 7, e41460 (2018).
Zeng, Y., Wagner, E. J. & Cullen, B. R. Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol. Cell 9, 1327–1333 (2002).
Imai, T. et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 4, 26238 (2015).
Wu, A. Y.-T. et al. Multiresolution imaging using bioluminescence resonance energy transfer identifies distinct biodistribution profiles of extracellular vesicles and exomeres with redirected tropism. Adv. Sci. 7, 2001467 (2020).
Driedonks, T. et al. Pharmacokinetics and biodistribution of extracellular vesicles administered intravenously and intranasally to Macaca nemestrina. J. Extracell. Biol. 1, e59 (2022).
Paget, D. et al. Comparative and integrated analysis of plasma extracellular vesicle isolation methods in healthy volunteers and patients following myocardial infarction. J. Extracell. Biol. 1, e66 (2022).
Visan, K. S. et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J. Extracell. Vesicles 11, e12266 (2022).
Hsia, T. et al. Rigorous comparison of extracellular vesicle processing to enhance downstream analysis for glioblastoma characterization. Adv. Biol. 8, e2300233 (2024).
Zhang, Z. et al. Comprehensive characterization of human brain-derived extracellular vesicles using multiple isolation methods: implications for diagnostic and therapeutic applications. J. Extracell. Vesicles 12, e12358 (2023).
Nieuwland, R., Siljander, P. R.-M., Falcón-Pérez, J. M. & Witwer, K. W. Reproducibility of extracellular vesicle research. Eur. J. Cell Biol. 101, 151226 (2022).
Poupardin, R. et al. Advances in extracellular vesicle research over the past decade: source and isolation method are connected with cargo and function. Adv. Healthc. Mater. 13, e2303941 (2024).
Abyadeh, M. et al. Proteomic profiling of mesenchymal stem cell-derived extracellular vesicles: impact of isolation methods on protein cargo. J. Extracell. Biol. 3, e159 (2024).
Han, C. et al. CD63-snorkel tagging for isolation of exosomes. Extracell. Vesicle 2, 100031 (2023).
Rufino-Ramos, D. et al. Using genetically modified extracellular vesicles as a non-invasive strategy to evaluate brain-specific cargo. Biomaterials 281, 121366 (2022).
Li, W. et al. Construction of a mouse model that can be used for tissue-specific EV screening and tracing in vivo. Front. Cell Dev. Biol. 10, 1015841 (2022).
Zheng, W. et al. Cell‐specific targeting of extracellular vesicles though engineering the glycocalyx. J. Extracell. Vesicles 11, e12290 (2022).
Liang, X. et al. Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models. J. Extracell. Vesicles 11, e12248 (2022).
Vogt, S. et al. An engineered CD81-based combinatorial library for selecting recombinant binders to cell surface proteins: laminin binding CD81 enhances cellular uptake of extracellular vesicles. J. Extracell. Vesicles 10, e12139 (2021).
Dooley, K. et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. 29, 1729–1743 (2021).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Martin Perez, C. et al. An extracellular vesicle delivery platform based on the PTTG1IP protein. Extracell. Vesicle 4, 100054 (2024).
Ohno, S. et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 21, 185–191 (2013).
Kehrloesser, S. et al. Cell-of-origin–specific proteomics of extracellular vesicles. PNAS Nexus 2, pgad107 (2023).
Kokkinopoulou, M., Simon, J., Landfester, K., Mailänder, V. & Lieberwirth, I. Visualization of the protein corona: towards a biomolecular understanding of nanoparticle-cell-interactions. Nanoscale 9, 8858–8870 (2017).
Wolf, M. et al. A functional corona around extracellular vesicles enhances angiogenesis, skin regeneration and immunomodulation. J. Extracell. Vesicles 11, e12207 (2022).
Choi, D. et al. Quantitative proteomic analysis of trypsin-treated extracellular vesicles to identify the real-vesicular proteins. J. Extracell. Vesicles 9, 1757209 (2020).
Xu, R. et al. Surfaceome of exosomes secreted from the colorectal cancer cell line SW480: peripheral and integral membrane proteins analyzed by proteolysis and TX114. Proteomics 19, e1700453 (2019).
Rai, A., Fang, H., Claridge, B., Simpson, R. J. & Greening, D. W. Proteomic dissection of large extracellular vesicle surfaceome unravels interactive surface platform. J. Extracell. Vesicles 10, e12164 (2021).
Santucci, L. et al. Biological surface properties in extracellular vesicles and their effect on cargo proteins. Sci. Rep. 9, 13048 (2019).
Kirkemo, L. L. et al. Cell-surface tethered promiscuous biotinylators enable comparative small-scale surface proteomic analysis of human extracellular vesicles and cells. eLife 11, e73982 (2022).
Chauhan, S. et al. Surface glycoproteins of exosomes shed by myeloid-derived suppressor cells contribute to function. J. Proteome Res. 16, 238–246 (2017).
Li, Y., Kanao, E., Yamano, T., Ishihama, Y. & Imami, K. TurboID-EV: proteomic mapping of recipient cellular proteins proximal to small extracellular vesicles. Anal. Chem. 95, 14159–14164 (2023).
Skotland, T., Sandvig, K. & Llorente, A. Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res. 66, 30–41 (2017).
Lee, B. R. et al. Ascorbate peroxidase-mediated in situ labelling of proteins in secreted exosomes. J. Extracell. Vesicles 11, e12239 (2022).
Hagey, D. W. et al. The cellular response to extracellular vesicles is dependent on their cell source and dose. Sci. Adv. 9, eadh1168 (2024).
Shipunova, V. O., Shilova, O. N., Shramova, E. I., Deyev, S. M. & Proshkina, G. M. A highly specific substrate for nanoLUC luciferase furimazine is toxic in vitro and in vivo. Russ. J. Bioorg. Chem. 44, 225–228 (2018).
Gaspar, N. et al. Evaluation of NanoLuc substrates for bioluminescence imaging of transferred cells in mice. J. Photochem. Photobiol. B 216, 112128 (2021).
van Solinge, T. S. et al. Illuminating cellular and extracellular vesicle-mediated communication via a split-Nanoluc reporter in vitro and in vivo. Cell Rep. Methods 3, 100412 (2023).
Leary, N. et al. Melanoma-derived extracellular vesicles mediate lymphatic remodelling and impair tumour immunity in draining lymph nodes. J. Extracell. Vesicles 11, e12197 (2022).
Men, Y. et al. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat. Commun. 10, 4136 (2019).
Hu, Y. et al. Cancer-cell-secreted miR-204-5p induces leptin signalling pathway in white adipose tissue to promote cancer-associated cachexia. Nat. Commun. 14, 5179 (2023).
Ma, S. et al. Skeletal muscle-derived extracellular vesicles transport glycolytic enzymes to mediate muscle-to-bone crosstalk. Cell Metab. 35, 2028–2043.e7 (2023).
Hyenne, V. et al. Studying the fate of tumor extracellular vesicles at high spatiotemporal resolution using the zebrafish embryo. Dev. Cell 48, 554–572.e7 (2019).
Verweij, F. J. et al. Live tracking of inter-organ communication by endogenous exosomes in vivo. Dev. Cell 48, 573–589.e4 (2019).
Chu, J. et al. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nat. Biotechnol. 34, 760–767 (2016).
Hikita, T., Miyata, M., Watanabe, R. & Oneyama, C. In vivo imaging of long-term accumulation of cancer-derived exosomes using a BRET-based reporter. Sci. Rep. 10, 16616 (2020).
Schaub, F. X. et al. Fluorophore-NanoLuc BRET reporters enable sensitive in vivo optical imaging and flow cytometry for monitoring tumorigenesis. Cancer Res. 75, 5023–5033 (2015).
Suzuki, K. et al. Five colour variants of bright luminescent protein for real-time multicolour bioimaging. Nat. Commun. 7, 13718 (2016).
Zhang, H. et al. Quantitative assessment of near-infrared fluorescent proteins. Nat. Methods 20, 1605–1616 (2023).
Liang, X. et al. Multimodal engineering of extracellular vesicles for efficient intracellular protein delivery. Preprint at bioRxiv https://doi.org/10.1101/2023.04.30.535834 (2023).
Zickler, A. M. et al. Novel endogenous engineering platform for robust loading and delivery of functional mRNA by extracellular vesicles. Adv. Sci. 11, e2407619 (2024).
Zomer, A. et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161, 1046–1057 (2015).
Casella, G. et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci. Transl Med. 12, eaba0599 (2020).
Kang, M., Jordan, V., Blenkiron, C. & Chamley, L. W. Biodistribution of extracellular vesicles following administration into animals: a systematic review. J. Extracell. Vesicles 10, e12085 (2021).
Kur, I.-M. et al. Neuronal activity triggers uptake of hematopoietic extracellular vesicles in vivo. PLoS Biol. 18, e3000643 (2020).
Ridder, K. et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 12, e1001874 (2014).
Valkov, N. et al. SnRNA sequencing defines signaling by RBC-derived extracellular vesicles in the murine heart. Life Sci. Alliance 4, e202101048 (2021).
Nikolic, J. et al. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat. Commun. 9, 1029 (2018).
de Jong, O. G. et al. A CRISPR-Cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA. Nat. Commun. 11, 1113 (2020).
Platt, R. J. et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 159, 440–455 (2014).
Zhang, L. et al. sgRNA knock-in mouse provides an alternative approach for in vivo genetic modification. Front. Cell Dev. Biol. 9, 769673 (2022).
Kranich, J. et al. In vivo identification of apoptotic and extracellular vesicle-bound live cells using image-based deep learning. J. Extracell. Vesicles 9, 1792683 (2020).
Joshi, B. S., de Beer, M. A., Giepmans, B. N. G. & Zuhorn, I. S. Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano 14, 4444–4455 (2020).
Votteler, J. et al. Designed proteins induce the formation of nanocage-containing extracellular vesicles. Nature 540, 292–295 (2016).
Qin, W. et al. Dynamic mapping of proteome trafficking within and between living cells by TransitID. Cell 186, 3307–3324.e30 (2023).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Atai, N. A. et al. Heparin blocks transfer of extracellular vesicles between donor and recipient cells. J. Neurooncol. 115, 343–351 (2013).
Zhang, W. et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev. Cell 57, 329–343.e7 (2022).
Franciszkiewicz, K. et al. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res. 73, 617–628 (2013).
Nolte-‘t Hoen, E. N. M., Buschow, S. I., Anderton, S. M., Stoorvogel, W. & Wauben, M. H. M. Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 113, 1977–1981 (2009).
Zapatero-Belinchón, F. J., Carriquí-Madroñal, B. & Gerold, G. in Advances in Virus Research Vol. 109 (ed. Gerold, G.) 63–104 (Academic, 2021).
Lu, S. et al. Native and engineered extracellular vesicles for wound healing. Front. Bioeng. Biotechnol. 10, 1053217 (2022).
de Abreu, R. C. et al. Native and bioengineered extracellular vesicles for cardiovascular therapeutics. Nat. Rev. Cardiol. 17, 685–697 (2020).
Zaborowski, M. P. et al. Membrane-bound Gaussia luciferase as a tool to track shedding of membrane proteins from the surface of extracellular vesicles. Sci. Rep. 9, 17387 (2019).
Ai, Y. et al. Endocytosis blocks the vesicular secretion of exosome marker proteins. Sci. Adv. 10, eadi9156 (2024).
Thompson, A. G. et al. Extracellular vesicles in neurodegenerative disease — pathogenesis to biomarkers. Nat. Rev. Neurol. 12, 346–357 (2016).
Boulanger, C. M., Loyer, X., Rautou, P.-E. & Amabile, N. Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 14, 259–272 (2017).
Grange, C. & Bussolati, B. Extracellular vesicles in kidney disease. Nat. Rev. Nephrol. 18, 499–513 (2022).
Buzas, E. I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 23, 236–250 (2023).
O’Brien, K., Breyne, K., Ughetto, S., Laurent, L. C. & Breakefield, X. O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 21, 585–606 (2020).
Wu, J. & Jaffrey, S. R. Imaging mRNA trafficking in living cells using fluorogenic proteins. Curr. Opin. Chem. Biol. 57, 177–183 (2020).
Lu, X., Kong, K. Y. S. & Unrau, P. J. Harmonizing the growing fluorogenic RNA aptamer toolbox for RNA detection and imaging. Chem. Soc. Rev. 52, 4071–4098 (2023).
Bonacquisti, E. E. et al. Fluorogenic RNA-based biomaterials for imaging and tracking the cargo of extracellular vesicles. J. Control. Release 374, 349–368 (2024).
Lindenbergh, M. F. S. & Stoorvogel, W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu. Rev. Immunol. 36, 435–459 (2018).
Shedden, K., Xie, X. T., Chandaroy, P., Chang, Y. T. & Rosania, G. R. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 63, 4331–4337 (2003).
Maugeri, M. et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 10, 4333 (2019).
Nawaz, M. et al. Lipid nanoparticles deliver the therapeutic VEGFA mRNA in vitro and in vivo and transform extracellular vesicles for their functional extensions. Adv. Sci. 10, e2206187 (2023).
Takahashi, A. et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 8, 15287 (2017).
Troyer, Z. et al. Extracellular vesicles carry SARS-CoV-2 spike protein and serve as decoys for neutralizing antibodies. J. Extracell. Vesicles 10, e12112 (2021).
Zhao, F. et al. Extracellular vesicles from Zika virus-infected cells display viral E protein that binds ZIKV-neutralizing antibodies to prevent infection enhancement. EMBO J. 42, e112096 (2023).
Gupta, D. et al. Amelioration of systemic inflammation via the display of two different decoy protein receptors on extracellular vesicles. Nat. Biomed. Eng. 5, 1084–1098 (2021).
Carotti, V., Rigalli, J. P., van Asbeck-van der Wijst, J. & Hoenderop, J. G. J. Interplay between purinergic signalling and extracellular vesicles in health and disease. Biochem. Pharmacol. 203, 115192 (2022).
Angioni, R. et al. CD73+ extracellular vesicles inhibit angiogenesis through adenosine A2B receptor signalling. J. Extracell. Vesicles 9, 1757900 (2020).
Sanderson, R. D., Bandari, S. K. & Vlodavsky, I. Proteases and glycosidases on the surface of exosomes: newly discovered mechanisms for extracellular remodeling. Matrix Biol. 75–76, 160–169 (2019).
An, Y. et al. Tumor exosomal ENPP1 hydrolyzes cGAMP to inhibit cGAS-STING signaling. Adv. Sci. 11, e230811 (2024).
Hansen, A. S. et al. T-cell derived extracellular vesicles prime macrophages for improved STING based cancer immunotherapy. J. Extracell. Vesicles 12, e12350 (2023).
Roefs, M. T. et al. Cardiac progenitor cell-derived extracellular vesicles promote angiogenesis through both associated- and co-isolated proteins. Commun. Biol. 6, 800 (2023).
Chen, G. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 (2018).
Poggio, M. et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell 177, 414–427.e13 (2019).
Zhong, W. et al. Tumor-derived small extracellular vesicles inhibit the efficacy of CAR T cells against solid tumors. Cancer Res. 83, 2790–2806 (2023).
Rausch, L. et al. Phosphatidylserine-positive extracellular vesicles boost effector CD8+ T cell responses during viral infection. Proc. Natl Acad. Sci. USA 120, e2210047120 (2023).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 (1996).
Blandin, A. et al. Extracellular vesicles are carriers of adiponectin with insulin-sensitizing and anti-inflammatory properties. Cell Rep. 42, 112866 (2023).
Jin, S. et al. Astroglial exosome HepaCAM signaling and ApoE antagonization coordinates early postnatal cortical pyramidal neuronal axon growth and dendritic spine formation. Nat. Commun. 14, 5150 (2023).
Seras-Franzoso, J. et al. Extracellular vesicles from recombinant cell factories improve the activity and efficacy of enzymes defective in lysosomal storage disorders. J. Extracell. Vesicles 10, e12058 (2021).
Harmati, M., Bukva, M., Böröczky, T., Buzás, K. & Gyukity-Sebestyén, E. The role of the metabolite cargo of extracellular vesicles in tumor progression. Cancer Metastasis Rev. 40, 1203–1221 (2021).
Zhang, Y., Liang, F., Zhang, D., Qi, S. & Liu, Y. Metabolites as extracellular vesicle cargo in health, cancer, pleural effusion, and cardiovascular diseases: an emerging field of study to diagnostic and therapeutic purposes. Biomed. Pharmacother. 157, 114046 (2023).
Pham, T. T. et al. Endocytosis of red blood cell extracellular vesicles by macrophages leads to cytoplasmic heme release and prevents foam cell formation in atherosclerosis. J. Extracell. Vesicles 12, e12354 (2023).
Liang, Y. et al. Cell-derived extracellular vesicles for CRISPR/Cas9 delivery: engineering strategies for cargo packaging and loading. Biomater. Sci. 10, 4095–4106 (2022).
Kostyushev, D. et al. Gene editing by extracellular vesicles. Int. J. Mol. Sci. 21, 7362 (2020).
Valadi, H. et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 (2007).
Zhang, C. et al. Intercellular mitochondrial component transfer triggers ischemic cardiac fibrosis. Sci. Bull. 68, 1784–1799 (2023).
Crewe, C. et al. Extracellular vesicle-based interorgan transport of mitochondria from energetically stressed adipocytes. Cell Metab. 33, 1853–1868.e11 (2021).
Liang, W. et al. Mitochondria are secreted in extracellular vesicles when lysosomal function is impaired. Nat. Commun. 14, 5031 (2023).
Borcherding, N. & Brestoff, J. R. The power and potential of mitochondria transfer. Nature 623, 283–291 (2023).
Li, X. et al. Extracellular vesicle–encapsulated adeno-associated viruses for therapeutic gene delivery to the heart. Circulation 148, 405–425 (2023).
Hirose, H., Hirai, Y., Sasaki, M., Sawa, H. & Futaki, S. Quantitative analysis of extracellular vesicle uptake and fusion with recipient cells. Bioconjug. Chem. 33, 1852–1859 (2022).
Bui, S., Dancourt, J. & Lavieu, G. Virus-free method to control and enhance extracellular vesicle cargo loading and delivery. ACS Appl. Bio Mater. 6, 1081–1091 (2023).
Hamilton, J. R. et al. In vivo human T cell engineering with enveloped delivery vehicles. Nat. Biotechnol. 42, 1684–1692 (2024).
Hamilton, J. R. et al. Targeted delivery of CRISPR-Cas9 and transgenes enables complex immune cell engineering. Cell Rep. 35, 109207 (2021).
Segel, M. et al. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 373, 882–889 (2021).
Acknowledgements
S.E.A. was funded by the European Research Council under the European Union’s Horizon 2020 research and innovation programme (EXPERT, grant agreement No. 825828), the European Research Council Consolidator Grant (DELIVER, grant agreement No. 101001374), the Swedish Foundation of Strategic Research (FormulaEx, grant agreement No. SM19-0007), the Swedish Cancer Society (project agreement No. 21-1762-Pj-01-H) and the Swedish Research Council (project agreement No. 4-258/2021). The authors acknowledge M. Mowoe for linguistic editing.
Author information
Authors and Affiliations
Contributions
W.Z. researched data for the draft and contributed to the writing and editing of this manuscript. S.R. and H.Z. contributed to the discussion of content, writing and editing of this manuscript. M.L.C. and G.v.N. contributed to reviewing and editing of the manuscript before submission. J.Z.N. made a substantial contribution to the discussion of content. S.E.A. contributed to the discussion and reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
J.Z.N. and S.E.A. are consultants for and have equity interests in EVOX Therapeutics, Oxford, UK. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Yongjie Yang, Dima Ter-Ovanesyan, who co-reviewed with David Walt, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, W., Roudi, S., Zhou, H. et al. Genetic tools for investigating the life cycle of extracellular vesicles. Nat Rev Bioeng 3, 505–520 (2025). https://doi.org/10.1038/s44222-025-00286-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-025-00286-6