Abstract
Aim:
Investigation into pharmacokinetic-pharmacodynamic properties of inter-feron-alpha (IFN-α)2b-loaded poly(lactic-co-glycolic acid) (PLGA) microspheres (MS) in rhesus monkey primates.
Method:
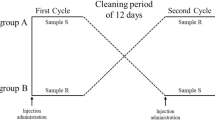
IFN-α2b was loaded with biodegradable PLGA with 3 inherent viscosities using a double emulsion and solvent evaporation method. The particle size, surface morphology, and in vitro release profiles were investigated. Two groups of rhesus monkeys (n=3) were injected intramuscularly with either 3 MlU/kg commercial IFN-α2b lyophilized powder or IFN-α2b-loaded PLGA microspheres (inherent viscosity of 0.89 dL/g). In vitro release was determined by Lowry protein assay. The serum IFN and neopterin levels were determined by the enzyme-linked immunosorbent assay (ELISA) method to evaluate biological activity of the microspheres in rhesus monkeys.
Results:
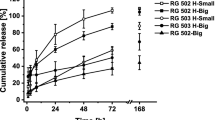
The IFN-α2b microspheres with 3 inherent viscosities (0.39, 0.89, and 1.13 dL/g) were entirely spherical and had a smooth surface. The average diameter of each type was 45.55, 81.23, and 110.25 urn, respectively. The in vitro release was 30 d. The pharmacokinetic-pharmacodynamic properties between the IFN-α2b microspheres and IFN-α2b lyophilized powder were significantly different (P<0.05).
Conclusion:
The drug residence time for the IFN-α2b of the PLGA microsphere with an inherent viscosity of 0.89 dL/g in plasma significantly increased and had a longer time of biological effects in rhesus monkeys following intramuscular administration.
Similar content being viewed by others
Article PDF
References
Férir G, Kaptein S, Neyts J, De Clercq E . Antiviral treatment of chronic hepatitis B virus infections: the past, the present and the future. Rev Med Virol 2008; 18: 19–34.
Yu ML, Huang CF, Dai CY, Huang JF, Chuang WL . Long-term effects of interferon-based therapy for chronic hepatitis C. Oncology 2007; 72: 16–23.
Uzé G, Schreiber G, Piehler J, Pellegrini S . The receptor of the type I interferon family. Curr Top Microbiol Immunol 2007; 316: 71–95.
Pogorzelska J, Flisiak R . Long-term effects of HBV treatment Peg-IFN alfa-2a alone or combined with lamivudine. Przegl Epidemiol 2007; 61: 433–7.
Zhou S, Deng X, He S, Li X, Jia W, Wei D, et al. Study on biodegradable microspheres containing recombinant interferon-alpha-2a. J Pharm Pharmacol 2002; 54: 1287–92.
Sánchez A, Tobío M, González L, Fabra A, Alonso MJ . Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-alpha. Eur J Pharm Sci 2003; 18: 221–9.
Krasowska D, Chodorowska G, Bartosińska J, Warmińska J, Jermak A, Kur A, et al. Serum levels of neopterin in patients with lichen planus. Ann Univ Mariae Curie Sklodowska 2004; 59: 346–50.
Xu AL, Yang F, Gao JP, Zhao YM, Wang L, Dong ML, inventors; Sun Yat-Sen University, Guangzhou. A method for preparation of Interferon-loaded biodegradable PLGA microspheres. China patent 1660412A. 2005 Aug 31.
Reich G . Ultrasound-induced degradation of PLA and PLGA during microsphere processing: influence of formulation variables. Eur J Pharm Biopharm 1998; 45: 165–71.
Cesur S . Neopterin: a marker used for monitoring infections. Mikrobiyol Bul 2005; 39: 251–60.
Lortkipanidze NT, Tevzadze MSh, Kamkamidze GK . Interferon-gamma and neopterin in alopecia areata. Georgian Med News 2005; ( 123): 53–7.
Iyer A, Hatta M, Usman R, Luiten S, Oskam L, Faber, W, et al. Serum levels of interferon-gamma, tumour necrosis factor-alpha, soluble interleukin-6R and soluble cell activation markers for monitoring response to treatment of leprosy reactions. Clin Exp Immunol 2007; 150: 210–6.
Brearley C, Jaber A, Bertolino M, Priestley A, Seiberling M . Assessment of the safety, tolerability, and PK/PD properties of two new formulations of subcutaneously administered IFN-betala: a double-blind, placebo-controlled comparison with the currently available formulation. Int J Clin Pharmacol Ther 2007; 45: 307–18.
Mager DE, Neuteboom B, Jusko WJ . Pharmacokinetics and pharmacodynamics of PEGylated IFN-beta la following subcutaneous administration in monkeys. Pharm Res 2005; 22: 58–61.
Pepinsky RB, LePage DJ, Gill A, Chakraborty A, Vaidyanathan S, Green M, et al. Improved pharmacokinetic properties of a polyethylene glycol-modified form of interferon-beta-1a with preserved in vitro bioactivity. J Pharmacol Exp Ther 2001; 297: 1059–66.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project supported by the Science and Technology Foundation of Guangdong Province (No 2006B35501006).
Rights and permissions
About this article
Cite this article
Zhang, Ym., Yang, F., Yang, Yq. et al. Recombinant interferon-alpha2b poly(lactic-co-glycolic acid) microspheres: pharmacokinetics-pharmacodynamics study in rhesus monkeys following intramuscular administration. Acta Pharmacol Sin 29, 1370–1375 (2008). https://doi.org/10.1111/j.1745-7254.2008.00881.x
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1111/j.1745-7254.2008.00881.x