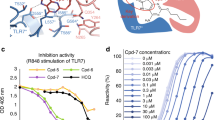
Abstract
Endosomal Toll-like receptors (TLR3, TLR7, TLR8, and TLR9) are highly analogous sensors for various viral or bacterial RNA and DNA molecular patterns. Nonetheless, few small molecules can selectively modulate these TLRs. In this manuscript, we identified the first human TLR8-specific small-molecule antagonists via a novel inhibition mechanism. Crystal structures of two distinct TLR8–ligand complexes validated a unique binding site on the protein–protein interface of the TLR8 homodimer. Upon binding to this new site, the small-molecule ligands stabilize the preformed TLR8 dimer in its resting state, preventing activation. As a proof of concept of their therapeutic potential, we have demonstrated that these drug-like inhibitors are able to suppress TLR8-mediated proinflammatory signaling in various cell lines, human primary cells, and patient specimens. These results not only suggest a novel strategy for TLR inhibitor design, but also shed critical mechanistic insight into these clinically important immune receptors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 22, 240–273 (2009).
Broz, P. & Monack, D.M. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 13, 551–565 (2013).
Pasare, C. & Medzhitov, R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect. 6, 1382–1387 (2004).
Akira, S., Uematsu, S. & Takeuchi, O. Pathogen recognition and innate immunity. Cell 124, 783–801 (2006).
Kawai, T. & Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 13, 460–469 (2007).
Carpenter, S. & O'Neill, L.A. How important are Toll-like receptors for antimicrobial responses? Cell. Microbiol. 9, 1891–1901 (2007).
Kanzler, H., Barrat, F.J., Hessel, E.M. & Coffman, R.L. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 13, 552–559 (2007).
Mohammad Hosseini, A., Majidi, J., Baradaran, B. & Yousefi, M. Toll-like receptors in the pathogenesis of autoimmune diseases. Adv. Pharm. Bull. 5 Suppl 1: 605–614 (2015).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Panter, G., Kuznik, A. & Jerala, R. Therapeutic applications of nucleic acids as ligands for Toll-like receptors. Curr. Opin. Mol. Ther. 11, 133–145 (2009).
Gantier, M.P. et al. TLR7 is involved in sequence-specific sensing of single-stranded RNAs in human macrophages. J. Immunol. 180, 2117–2124 (2008).
Gorden, K.B. et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J. Immunol. 174, 1259–1268 (2005).
Heil, F. et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
Hemmi, H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3, 196–200 (2002).
Papadimitraki, E.D., Bertsias, G.K. & Boumpas, D.T. Toll like receptors and autoimmunity: a critical appraisal. J. Autoimmun. 29, 310–318 (2007).
Barrat, F.J. et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 202, 1131–1139 (2005).
Marshak-Rothstein, A. Toll-like receptors in systemic autoimmune disease. Nat. Rev. Immunol. 6, 823–835 (2006).
Watanabe, M. et al. Dihydropyrrolo[2,3-d]pyrimidines: selective toll-like receptor 9 antagonists from scaffold morphing efforts. ACS Med. Chem. Lett. 5, 1235–1239 (2014).
Cheng, K., Wang, X. & Yin, H. Small-molecule inhibitors of the TLR3/dsRNA complex. J. Am. Chem. Soc. 133, 3764–3767 (2011).
Hennessy, E.J., Parker, A.E. & O'Neill, L.A. Targeting Toll-like receptors: emerging therapeutics? Nat. Rev. Drug Discov. 9, 293–307 (2010).
Kondo, T., Kawai, T. & Akira, S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 33, 449–458 (2012).
Botos, I., Segal, D.M. & Davies, D.R. The structural biology of Toll-like receptors. Structure 19, 447–459 (2011).
Tanji, H., Ohto, U., Shibata, T., Miyake, K. & Shimizu, T. Structural reorganization of the Toll-like receptor 8 dimer induced by agonistic ligands. Science 339, 1426–1429 (2013).
Tanji, H. et al. Toll-like receptor 8 senses degradation products of single-stranded RNA. Nat. Struct. Mol. Biol. 22, 109–115 (2015).
Kokatla, H.P. et al. Exquisite selectivity for human toll-like receptor 8 in substituted furo[2,3-c]quinolines. J. Med. Chem. 56, 6871–6885 (2013).
Salunke, D.B. et al. Structure-activity relationships in human Toll-like receptor 8-active 2,3-diamino-furo[2,3-c]pyridines. J. Med. Chem. 55, 8137–8151 (2012).
Kuznik, A. et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 186, 4794–4804 (2011).
Schön, M.P. & Schön, M. TLR7 and TLR8 as targets in cancer therapy. Oncogene 27, 190–199 (2008).
Lamphier, M. et al. Novel small molecule inhibitors of TLR7 and TLR9: mechanism of action and efficacy in vivo. Mol. Pharmacol. 85, 429–440 (2014).
Liu, H., Liu, Z.H., Chen, Z.H., Yang, J.W. & Li, L.S. Triptolide: a potent inhibitor of NF-kappa B in T-lymphocytes. Acta Pharmacol. Sin. 21, 782–786 (2000).
Jurk, M. et al. Human TLR7 or TLR8 independently confer responsiveness to the antiviral compound R-848. Nat. Immunol. 3, 499 (2002).
Yin, H. & Flynn, A.D. Drugging membrane protein interactions. Annu. Rev. Biomed. Eng. 18, 51–76 (2016).
Beesu, M. et al. Structure-based design of human TLR8-specific agonists with augmented potency and adjuvanticity. J. Med. Chem. 58, 7833–7849 (2015).
Valencia Pacheco, G.J. et al. Expression and activation of intracellular receptors TLR7, TLR8 and TLR9 in peripheral blood monocytes from HIV-infected patients. Colomb. Med. 44, 92–99 (2013).
Zhang, Z. et al. Structural analysis reveals that toll-like receptor 7 Is a dual receptor for guanosine and single-stranded RNA. Immunity 45, 737–748 (2016).
Kaczanowska, S., Joseph, A.M. & Davila, E. TLR agonists: our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 93, 847–863 (2013).
Doyle, S. et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17, 251–263 (2002).
Tseng, P.H. et al. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nat. Immunol. 11, 70–75 (2010).
Duffy, L. & O'Reilly, S.C. Toll-like receptors in the pathogenesis of autoimmune diseases: recent and emerging translational developments. ImmunoTargets Ther. 5, 69–80 (2016).
Liu, J. et al. A five-amino-acid motif in the undefined region of the TLR8 ectodomain is required for species-specific ligand recognition. Mol. Immunol. 47, 1083–1090 (2010).
Mullen, L., Ferdjani, J. & Sacre, S. Simvastatin inhibits Toll-like receptor 8 (TLR8) signaling in primary human monocytes and spontaneous tumor necrosis factor production from rheumatoid synovial membrane cultures. Mol. Med. 21, 726–734 (2015).
Sacre, S.M. et al. Inhibitors of TLR8 reduce TNF production from human rheumatoid synovial membrane cultures. J. Immunol. 181, 8002–8009 (2008).
Castañeda, S., Blanco, R. & González-Gay, M.A. Adult-onset Still's disease: advances in the treatment. Best Pract. Res. Clin. Rheumatol. 30, 222–238 (2016).
Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1, 135–145 (2001).
Piccinini, A.M. & Midwood, K.S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010, 672395 (2010).
Guiducci, C. et al. RNA recognition by human TLR8 can lead to autoimmune inflammation. J. Exp. Med. 210, 2903–2919 (2013).
Cheng, K. et al. Specific activation of the TLR1-TLR2 heterodimer by small-molecule agonists. Sci. Adv. 1, e1400139 (2015).
Csakai, A. et al. Saccharin derivatives as inhibitors of interferon-mediated inflammation. J. Med. Chem. 57, 5348–5355 (2014).
Das, N. et al. HMGB1 activates proinflammatory signaling via TLR5 leading to allodynia. Cell Rep. 17, 1128–1140 (2016).
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997).
Battye, T.G., Kontogiannis, L., Johnson, O., Powell, H.R. & Leslie, A.G. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 67, 271–281 (2011).
Vagin, A. & Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 66, 22–25 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Murshudov, G.N., Vagin, A.A. & Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 (1997).
Brennan, F.M., Chantry, D., Jackson, A.M., Maini, R.N. & Feldmann, M. Cytokine production in culture by cells isolated from the synovial membrane. J. Autoimmun. 2 (Suppl.) 177–186 (1989).
Ulmer, A.J., Scholz, W., Ernst, M., Brandt, E. & Flad, H.D. Isolation and subfractionation of human peripheral blood mononuclear cells (PBMC) by density gradient centrifugation on Percoll. Immunobiology 166, 238–250 (1984).
Acknowledgements
This work was funded by the National Institute of Health (NIH R01GM101279 to H.Y.), National Natural Science Foundation of China (No. 21572114 to H.Y. and No. 81401333 to J.L.), the University Key Scientific Research Program Foundation of Henan Province (No. 5201039140120 to S.Z.) and Grant-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology (H.T., U.O., and T.S.), CREST JST (T.S.), the Takeda Science Foundation (U.O. and T.S.), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (U.O.), the Naito Foundation (U.O.), and the Daiichi Sankyo Foundation of Life Science (U.O.). We thank X. Wang and W. Wang for their assistance with high-throughput screening and data analysis. We thank J. Dragavon for his assistance with confocal microscopy. We thank Y. Yamada and A. Shinoda for automated data collection at Photon Factory.
Author information
Authors and Affiliations
Contributions
H.Y. designed the project and supervised data analysis. S.Z. designed the experiments in consultation with H.Y. S.Z. performed the cell line establishment and high throughput screening. S.Z. and Z.H. performed chemical synthesis of compounds, cell culture and cellular inhibition studies. S.Z., Z.H., S.J., and N.D. performed immunoblotting experiments. H.T., K.S., U.O., and T.S. expressed protein, solved the crystal structure, performed isothermal titration calorimetry and gel-filtration experiments. J.L., J.J., and Y.B. contributed to the patient PBMC and synovial tissues extraction. S.J. performed PBMC and synovial cells extraction. S.J. and S.Z. performed primary cell and patient specimen studies. H.Y. and S.Z. wrote the manuscript with input from Z.H., S.J., and H.T.
Corresponding authors
Ethics declarations
Competing interests
H.Y., S.Z., and Z.H. have filed a patent application based on the technology reported in this manuscript.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1–4, Supplementary Figures 1–17 (PDF 2115 kb)
Supplementary Note 1
Chemical synthesis and characterizations (PDF 623 kb)
Rights and permissions
About this article
Cite this article
Zhang, S., Hu, Z., Tanji, H. et al. Small-molecule inhibition of TLR8 through stabilization of its resting state. Nat Chem Biol 14, 58–64 (2018). https://doi.org/10.1038/nchembio.2518
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2518
This article is cited by
-
Erythroid-intrinsic activation of TLR8 impairs erythropoiesis in inherited anemia
Nature Communications (2024)
-
Label-free biosensor assay decodes the dynamics of Toll-like receptor signaling
Nature Communications (2024)
-
Recognition of Mycobacterium tuberculosis by macrophage Toll-like receptor and its role in autophagy
Inflammation Research (2024)
-
naRNA-LL37 composite DAMPs define sterile NETs as self-propagating drivers of inflammation
EMBO Reports (2024)
-
Modulation of IRAK enzymes as a therapeutic strategy against SARS-CoV-2 induced cytokine storm
Clinical and Experimental Medicine (2023)