Key Points
-
RNA viruses, which are most common in eukaryotes, are among the simplest forms of life.
-
Genomic and metagenomic studies have highlighted remarkable diversity of a major class of RNA viruses, the extended picornavirus-like superfamily.
-
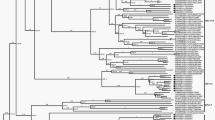
Phylogenetic analysis reveals close evolutionary relationships between RNA viruses infecting unicellular eukaryotes and distinct families of picorna-like viruses of plants and animals.
-
This suggests that diversification of picorna-like viruses antedated radiation of the eukaryotes and probably occurred in a 'Big Bang' concomitant with the key events of eukaryogenesis.
-
The origins of the conserved genes of picorna-like viruses can be traced to specific prokaryotic ancestors.
-
The Big Bang of picorna-like virus evolution might have been triggered by chance assembly of these ancestral genes at the earliest stages of eukaryogenesis.
Abstract
The recent discovery of RNA viruses in diverse unicellular eukaryotes and developments in evolutionary genomics have provided the means for addressing the origin of eukaryotic RNA viruses. The phylogenetic analyses of RNA polymerases and helicases presented in this Analysis article reveal close evolutionary relationships between RNA viruses infecting hosts from the Chromalveolate and Excavate supergroups and distinct families of picorna-like viruses of plants and animals. Thus, diversification of picorna-like viruses probably occurred in a 'Big Bang' concomitant with key events of eukaryogenesis. The origins of the conserved genes of picorna-like viruses are traced to likely ancestors including bacterial group II retroelements, the family of HtrA proteases and DNA bacteriophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Joyce, G. F. The antiquity of RNA-based evolution. Nature 418, 214–221 (2002). In-depth analysis of the RNA world concept of the primordial genetic systems.
Koonin, E. V. & Martin, W. On the origin of genomes and cells within inorganic compartments. Trends Genet. 21, 647–654 (2005). A conceptual framework for the origin of life within microscopic mineral compartments at hydrothermal vents through Darwinian selection of self-replicating, recombining RNA molecules that gradually evolved into complex molecular ensembles.
Holmes, E. C. & Drummond, A. J. The evolutionary genetics of viral emergence. Curr. Top. Microbiol. Immunol. 315, 51–66 (2007).
Suttle, C. A. Viruses in the sea. Nature 437, 356–361 (2005).
Edwards, R. A. & Rohwer, F. Viral metagenomics. Nature Rev. Microbiol. 3, 504–510 (2005).
Angly, F. E. et al. The marine viromes of four oceanic regions. PLoS Biol. 4, e368 (2006).
Suttle, C. A. Marine viruses — major players in the global ecosystem. Nature Rev. Microbiol. 5, 801–812 (2007). This incisive review provides a broad prospective on the abundance, diversity and role of the marine viruses in the biosphere.
Prangishvili, D., Garrett, R. A. & Koonin, E. V. Evolutionary genomics of archaeal viruses: unique viral genomes in the third ___domain of life. Virus Res. 117, 52–67 (2006).
Khayat, R. et al. Structure of an archaeal virus capsid protein reveals a common ancestry to eukaryotic and bacterial viruses. Proc. Natl Acad. Sci. USA 102, 18944–18949 (2005).
Ortmann, A. C., Wiedenheft, B., Douglas, T. & Young, M. Hot crenarchaeal viruses reveal deep evolutionary connections. Nature Rev. Microbiol. 4, 520–528 (2006).
Nandhagopal, N. et al. The structure and evolution of the major capsid protein of a large, lipid-containing DNA virus. Proc. Natl Acad. Sci. USA 99, 14758–14763 (2002).
Dunigan, D. D., Fitzgerald, L. A. & Van Etten, J. L. Phycodnaviruses: a peek at genetic diversity. Virus Res. 117, 119–132 (2006).
Dupuy, C., Huguet, E. & Drezen, J. M. Unfolding the evolutionary story of polydnaviruses. Virus Res. 117, 81–89 (2006).
Raoult, D. et al. The 1.2-megabase genome sequence of Mimivirus. Science 306, 1344–1350 (2004).
Claverie, J. M. et al. Mimivirus and the emerging concept of “giant” virus. Virus Res. 117, 133–144 (2006).
Hendrix, R. W. Bacteriophage genomics. Curr. Opin. Microbiol. 6, 506–511 (2003).
Casjens, S. R. Comparative genomics and evolution of the tailed-bacteriophages. Curr. Opin. Microbiol. 8, 451–458 (2005).
Bamford, D. H., Grimes, J. M. & Stuart, D. I. What does structure tell us about virus evolution? Curr. Opin. Struct. Biol. 15, 655–663 (2005). Homologous capsid proteins are seen in a wide variety of superficially unrelated icosahedral viruses that infect diverse hosts, in a striking demonstration of far-reaching evolutionary connections between viruses.
Liu, J., Glazko, G. & Mushegian, A. Protein repertoire of double-stranded DNA bacteriophages. Virus Res. 117, 68–80 (2006).
Pedulla, M. L. et al. Origins of highly mosaic mycobacteriophage genomes. Cell 113, 171–182 (2003).
Sullivan, M. B. et al. Prevalence and evolution of core photosystem II genes in marine cyanobacterial viruses and their hosts. PLoS Biol. 4, e234 (2006).
Iyer, L. M., Balaji, S., Koonin, E. V. & Aravind, L. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 117, 156–184 (2006).
Claverie, J. M. Viruses take center stage in cellular evolution. Genome Biol. 7, 110 (2006).
Forterre, P. The origin of viruses and their possible roles in major evolutionary transitions. Virus Res. 117, 5–16 (2006).
Forterre, P. Three RNA cells for ribosomal lineages and three DNA viruses to replicate their genomes: a hypothesis for the origin of cellular ___domain. Proc. Natl Acad. Sci. USA 103, 3669–3674 (2006). A hypothesis that implicates viruses in the independent origins of the DNA replication machineries of the three domains of cellular life.
Gorinsek, B., Gubensek, F. & Kordis, D. Phylogenomic analysis of chromoviruses. Cytogenet. Genome Res. 110, 543–552 (2005).
Koonin, E. V. & Dolja, V. V. Evolution of complexity in the viral world: the dawn of a new vision. Virus Res. 117, 1–4 (2006).
Koonin, E. V., Senkevich, T. G. & Dolja, V. V. The ancient virus world and evolution of cells. Biol. Direct 1, 29 (2006). This article developed the concept of 'viral hallmark genes' — genes that are present in a variety of viruses but not in cellular life forms — and proposed that these genes comprise an uninterrupted flow of genetic information from pre-cellular stages of evolution to this day.
Pritham, E. J., Putliwala, T. & Feschotte, C. Mavericks, a novel class of giant transposable elements widespread in eukaryotes and related to DNA viruses. Gene 390, 3–17 (2007).
Raoult, D. & Forterre, P. Redefining viruses: lessons from Mimivirus. Nature Rev. Microbiol. 6, 315–319 (2008). A new definition of viruses capitalizes on the sharp distinction between viruses as capsid-encoding organisms and cellular life forms as ribosome-encoding organisms.
Hull, R. Matthews' Plant Virology (Academic Press, San Diego, 2001).
Knipe, D. M. & Howley, P. M. Fields Virology (Lippincott Williams & Wilkins, Philadelphia, 2001).
Koonin, E. V. & Dolja, V. V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit. Rev. Biochem. Mol. Biol. 28, 375–430 (1993). A conceptual synthesis on the early studies in comparative genomics and evolution of positive-strand RNA viruses; advances the concept of the three major superfamilies of the positive-strand RNA viruses.
Bollback, J. P. & Huelsenbeck, J. P. Phylogeny, genome evolution, and host specificity of single-stranded RNA bacteriophage (family Leviviridae). J. Mol. Evol. 52, 117–128 (2001).
Ruokoranta, T. M., Grahn, A. M., Ravantti, J. J., Poranen, M. M. & Bamford, D. H. Complete genome sequence of the broad host range single-stranded RNA phage PRR1 places it in the Levivirus genus with characteristics shared with Alloleviviruses. J. Virol. 80, 9326–9330 (2006).
Lang, A. S., Culley, A. I. & Suttle, C. A. Genome sequence and characterization of a virus (HaRNAV) related to picorna-like viruses that infects the marine toxic bloom-forming alga Heterosigma akashiwo. Virology 320, 206–217 (2004).
Nagasaki, K. et al. Comparison of genome sequences of single-stranded RNA viruses infecting the bivalve-killing dinoflagellate Heterocapsa circularisquama. Appl. Environ. Microbiol 71, 8888–8894 (2005).
Takao, Y., Mise, K., Nagasaki, K., Okuno, T. & Honda, D. Complete nucleotide sequence and genome organization of a single-stranded RNA virus infecting the marine fungoid protist Schizochytrium sp. J. Gen. Virol. 87, 723–733 (2006).
Shirai, Y. et al. Genomic and phylogenetic analysis of a single-stranded RNA virus infecting Rhizosolenia setigera (Stramenopiles: Baccilariophyceae). J. Mar. Biol. Ass. UK 86, 475–483 (2006).
Culley, A. I., Lang, A. S. & Suttle, C. A. High diversity of unknown picorna-like viruses in the sea. Nature 424, 1054–1057 (2003).
Culley, A. I., Lang, A. S. & Suttle, C. A. Metagenomic analysis of coastal RNA virus communities. Science 312, 1795–1798 (2006). This article uses the power of metagenomics to address diversity and evolutionary affinities of uncultured marine RNA viruses.
Culley, A. I., Lang, A. S. & Suttle, C. A. The complete genomes of three viruses assembled from shotgun libraries of marine RNA virus communities. Virol. J. 4 (2007).
Culley, A. I. & Steward, G. F. New genera of RNA viruses in subtropical seawater, inferred from polymerase gene sequences. Appl. Environ. Microbiol. 73, 5937–5944 (2007).
Koonin, E. V. The Biological Big Bang model for the major transitions in evolution. Biol. Direct 2, 21 (2007). A unifying concept of the major transitions in evolution as episodes of explosive diversification powered by rampant gene exchange and recombination.
Domingo, E., Escarmis, C., Mendez-Arias, L. & Holland, J. J. in Origin and Evolution of Viruses (eds Domingo, E., Webster, R. & Holland, J.) 141–161 (Academic Press, San Diego, 1999).
Gromeier, M., Wimmer, E. & Gorbalenya, A. E. in Origin and Evolution of Viruses (eds Domingo, E., Webster, R. & Holland, J.) 287–344 (Academic Press, San Diego, 1999).
Crotty, S., Cameron, C. E. & Andino, R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc. Natl Acad. Sci. USA 98, 6895–6900 (2001).
Domingo, E. et al. Viruses as quasispecies: biological implications. Curr. Top. Microbiol. Immunol. 299, 51–82 (2006). A recent review that emphasizes the significance of quasispecies for the adaptability and pathogenesis of RNA viruses and the ongoing evolution of the viral populations.
Vignuzzi, M., Stone, J. K., Arnold, J. J., Cameron, C. E. & Andino, R. Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439, 344–348 (2006).
Domingo, E., Martin, V., Perales, C. & Escarmis, C. Coxsackieviruses and quasispecies theory: evolution of enteroviruses. Curr. Top. Microbiol. Immunol. 323, 3–32 (2008).
Biebricher, C. K. & Eigen, M. What is a quasispecies? Curr. Top. Microbiol. Immunol. 299, 1–31 (2006). A broad analysis of the quasispecies concept and its application to the rapidly evolving RNA viruses.
Koonin, E. V. & Gorbalenya, A. E. Evolution of RNA genomes: does the high mutation rate necessitate high rate of evolution of viral proteins? J. Mol. Evol. 28, 524–527 (1989).
Goldbach, R. Genome similarities between plant and animal RNA viruses. Microbiol. Sci. 4, 197–202 (1987). The beginnings of the concept of superfamilies of positive-strand RNA viruses that span wide ranges of hosts.
Goldbach, R. & Wellink, J. Evolution of plus-strand RNA viruses. Intervirology 29, 260–267 (1988).
Koonin, E. V. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J. Gen. Virol. 72 (Pt 9), 2197–2206 (1991).
Zanotto, P. M., Gibbs, M. J., Gould, E. A. & Holmes, E. C. A reevaluation of the higher taxonomy of viruses based on RNA polymerases. J. Virol. 70, 6083–6096 (1996).
Strauss, E. G., Strauss, J. H. & Levine, A. J. in Fields Virology (eds Fields, B. N., Knipe, D. M. & Howley, P. M.) 153–171 (Lippincott-Raven, Philadelphia, 1996).
Gibbs, M. J., Koga, R., Moriyama, H., Pfeiffer, P. & Fukuhara, T. Phylogenetic analysis of some large double-stranded RNA replicons from plants suggests they evolved from a defective single-stranded RNA virus. J. Gen. Virol. 81, 227–233 (2000).
Gorbalenya, A. E., Enjuanes, L., Ziebuhr, J. & Snijder, E. J. Nidovirales: evolving the largest RNA virus genome. Virus Res. 117, 17–37 (2006).
Johnson, K. N., Johnson, K. L., Dasgupta, R., Gratsch, T. & Ball, A. L. Comparisons among the larger genome segments of six nodaviruses and their encoded RNA replicases. J. Gen. Virol. 82, 1855–1866 (2001).
Koonin, E. V. Evolution of double-stranded RNA viruses: a case for polyphyletic origin from different groups of positive-stranded RNA viruses. Semin. Virol. 3, 327–339 (1992).
Koonin, E. V., Gorbalenya, A. E. & Chumakov, K. M. Tentative identification of RNA-dependent RNA polymerases of dsRNA viruses and their relationship to positive strand RNA viral polymerases. FEBS Lett. 252, 42–46 (1989).
Gorbalenya, A. E. et al. The palm subdomain-based active site is internally permuted in viral RNA-dependent RNA polymerases of an ancient lineage. J. Mol. Biol. 324, 47–62 (2002).
Ahlquist, P. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nature Rev. Microbiol. 4, 371–382 (2006). A recent perspective on structural, functional and mechanistic similarities in replication of the diverse viruses that have RNA genomes.
Keeling, P. J. et al. The tree of eukaryotes. Trends Ecol. Evol. 20, 670–676 (2005). A conceptually important overview of eukaryotic evolution that introduces five supergroups, the exact relationships between which are difficult to determine.
Keeling, P. J. Genomics. Deep questions in the tree of life. Science 317, 1875–1876 (2007).
Hacker, C. V., Brasier, C. M. & Buck, K. W. A double-stranded RNA from a Phytophtora species is related to the plant endornaviruses and contains a putative UDP glycosyltransferase gene. J. Gen. Virol. 86, 1561–1570 (2005).
Le Gall, O. et al. Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T = 3 virion architecture. Arch. Virol. 153, 715–727 (2008). A formal description of the proposed order Picornavirales that comprises the core of the picorna-like virus superfamily.
Lima-Mendez, G., Van Helden, J., Toussaint, A. & Leplae, R. Reticulate representation of evolutionary and functional relationships between phage genomes. Mol. Biol. Evol. 25, 762–777 (2008).
Gordon, K. H. J. & Waterhouse, P. M. Small RNA viruses of insects: expression in plants and RNA silencing. Adv. Virus Res. 68, 459–502 (2006).
Van der Wilk, F., Dullemans, A. M., Verbeek, M. & Van der Heuvel, J. F. J. M. Nucleotide sequence and genomic organization of Acyrthosiphon pisum virus. Virology 238, 353–362 (1997).
Habayeb, M. S., Ekengren, S. K. & Hultmark, D. Nora virus, a persistent virus in Drosophila, defines a new picorna-like family. J. Gen. Virol. 87, 3045–3051 (2006).
Revill, P. A., Davidson, A. D. & Wright, P. J. The nucleotide sequence and genome organization of mushroom bacilliform virus. Virology 202, 904–911 (1994).
Yokoi, T., Takemoto, Y., Suzuki, M., Yamashita, S. & Hibi, T. The nucleotide sequence and genome organization of Sclerophtora macrospora virus B. Virology 264, 344–349 (1999).
Yokoi, T., Yamashita, S. & Hibi, T. The nucleotide sequence and genome organization of Sclerophtora macrospora virus A. Virology 311, 394–399 (2003).
Matsui, S. M. & Greenberg, H. B. in Fields Virology (eds Knipe, D. M. & Howley, P. M.) 875–893 (Lippncott Williams & Wilkins, Philadelphia, 2001).
Koonin, E. V., Choi, G. H., Nuss, D. L., Shapira, R. & Carrington, J. C. Evidence for common ancestry of a chestnut blight hypovirulence-associated double-stranded RNA and a group of positive-strand RNA plant viruses. Proc. Natl Acad. Sci. USA 88, 10647–10651 (1991).
Nuss, D. L. Hypovirulence: mycoviruses at the fungal-plant interface. Nature Rev. Microbiol. 3, 632–642 (2005). This article provides conceptual analysis of the interactions between viruses and their plant pathogenic fungal hosts.
Linder-Basso, D., Dynek, J. N. & Hillman, B. I. Genome analysis of Cryphonectria hypovirus 4, the most common hypovirus species in North America. Virology 337, 192–203 (2005).
Chu, Y. M. et al. Double-stranded RNA mycovirus from Fusarium graminearum. Appl. Environ. Microbiol. 68, 2529–2534 (2002).
Jiang, B., Monroe, S. S., Koonin, E. V., Stine, S. E. & Glass, R. I. RNA sequence of astrovirus: distinctive genomic organization and a putative retrovirus-like ribosomal frameshifting signal that directs the viral replicase synthesis. Proc. Natl. Acad. Sci. USA 90, 10539–10543 (1993).
Green, K. Y., Chanock, R. M. & Kapikian, A. Z. in Fields Virology (eds. Knipe, D. M. & Howley, P. M.) 841–874 (Lippincott Williams & Wilkins, Philadelphia, 2001).
Ghabrial, S. A. Origin, adaptation and evolutionary pathways of fungal viruses. Virus Genes 16, 119–131 (1998).
Caston, J. R. et al. Three-dimentional structure and stoichometry of Helmintosporium victroriae 190S totivirus. Virology 347, 323–332 (2006).
Khramtsov, N. V. & Upton, S. J. Association of RNA polymerase complexes of the parasitic protozoan Cryptosporidium parvum with virus-like particles: heterogeneous system. J. Virol. 74, 5788–5795 (2000).
Koga, R., Horiuchi, H. & Fukuhara, T. Double-stranded RNA replicons associated with chloroplasts of a green alga, Bryopsis cinicola. Plant Mol. Biol. 51, 991–999 (2003).
Valles, S. M., Strong, C. A. & Hashimoto, Y. A new positive-strand RNA virus with unique genome characteristics from the red imported fire ant, Solenopsis invicta. Virology 365, 457–463 (2007).
Gorbalenya, A. E., Donchenko, A. P., Blinov, V. M. & Koonin, E. V. Cysteine proteases of positive strand RNA viruses and chymotrypsin-like serine proteases. A distinct protein superfamily with a common structural fold. FEBS Lett. 243, 103–114 (1989). The first demonstration of a highly significant sequence similarity between picornaviral 3CPros and the HtrA family of bacterial proteases.
Crawford, L. J. et al. Molecular characterization of a partitivirus from Ophiostoma himal-ulmi. Virus Genes 33, 33–39 (2006).
Embley, T. M. & Martin, W. Eukaryotic evolution, changes and challenges. Nature 440, 623–630 (2006). A comprehensive review of the current concepts of the origin of the eukaryotic cell that makes the sharp distinction between symbiotic and archezoan scenarios.
Martin, W. & Koonin, E. V. Introns and the origin of nucleus–cytosol compartmentation. Nature 440, 41–45 (2006). A hypothesis of the major role of the invasion of group II introns as the principal driving force behind the emergence of the nucleus during eukaryogenesis.
Martin, W. & Muller, M. The hydrogen hypothesis for the first eukaryote. Nature 392, 37–41 (1998).
Rivera, M. C. & Lake, J. A. The ring of life provides evidence for a genome fusion origin of eukaryotes. Nature 431, 152–155 (2004). An original method of phylogenetic analysis provides evidence in support of the origin of eukaryotic cell through fusion of prokaryotic genomes.
Kurland, C. G., Collins, L. J. & Penny, D. Genomics and the irreducible nature of eukaryote cells. Science 312, 1011–1014 (2006).
Poole, A. & Penny, D. Eukaryote evolution: engulfed by speculation. Nature 447 913 (2007).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Poch, O., Sauvaget, I., Delarue, M. & Tordo, N. Identification of four conserved motifs among the RNA-dependent polymerase encoding elements. EMBO J. 8, 3867–3874 (1989). The first clear demonstration of structural and evolutionary relationships between viral RdRps and reverse transcriptases.
Ago, H. et al. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Structure 7, 1417–1426 (1999).
Hansen, J. L., Long, A. M. & Schultz, S. C. Structure of the RNA-dependent RNA polymerase of poliovirus. Structure 5, 1109–1122 (1997).
Ng, K. K., Arnold, J. J. & Cameron, C. E. Structure-function relationships among RNA-dependent RNA polymerases. Curr. Top. Microbiol. Immunol. 320, 137–156 (2008).
Arnold, J. J., Ghosh, S. K. & Cameron, C. E. Poliovirus RNA-dependent RNA polymerase (3Dpol). Divalent cation modulation of primer, template, and nucleotide selection. J. Biol. Chem. 274, 37060–37069 (1999).
Arnold, J. J., Gohara, D. W. & Cameron, C. E. Poliovirus RNA-dependent RNA polymerase (3Dpol): pre-steady-state kinetic analysis of ribonucleotide incorporation in the presence of Mn2+. Biochemistry 43, 5138–5148 (2004).
Hung., M., Gibbs, C. S. & Tsiang, M. Biochemical characterization of rhinovirus RNA-dependent RNA polymerase. Antiviral Res. 56, 99–114 (2002).
Lambowitz, A. M. & Zimmerly, S. Mobile group II introns. Annu. Rev. Genet. 38, 1–35 (2004). This article reviews the mechanistic and evolutionary aspects of group II introns that were implicated in the origin of spliceosomal introns.
Robart, A. R. & Zimmerly, S. Group II intron retroelements: function and diversity. Cytogenet. Genome Res. 110, 589–597 (2005).
Toor, N., Keating, K. S., Taylor, S. D. & Pyle, A. M. Crystal structure of a self-spliced group II intron. Science 320, 77–82 (2008). This article reviews the mechanistic and evolutionary aspects of group II introns that were implicated in the origin of spliceosomal introns.
Eickbush, T. H. & Jamburunthugoda, V. K. The diversity of retrotransposons and the properties of their reverse transcriptases. Virus Res. 134, 221–234 (2008).
Arkhipova, I. R., Pyatkov, K. I., Meselson, M. & Evgen'ev, M. B. Retroelements containing introns in diverse invertebrate taxa. Nature Genet. 33, 123–124 (2003).
Gladyshev, E. A. & Arkhipova, I. R. Telomere-associated endonuclease-deficient Penelope-like retroelements in diverse eukaryotes. Proc. Natl. Acad. Sci. USA 104, 9352–9357 (2007).
Clausen, T., Southan, C. & Ehrmann, M. The HtrA family of proteases: implications for protein composition and cell fate. Mol. Cell 10, 443–455 (2002).
Li, W. et al. Structural insights into the pro-apoptotic function of mitochondrial serine protease HtrA2/Omi. Nature Struct. Biol. 9, 436–441 (2002).
Gorbalenya, A. E., Koonin, E. V. & Wolf, Y. I. A new superfamily of putative NTP-binding domains encoded by genomes of small DNA and RNA viruses. FEBS Lett. 262, 145–148 (1990).
Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 (1999).
Iyer, L. M., Leipe, D. D., Koonin, E. V. & Aravind, L. Evolutionary history and higher order classification of AAA+ ATPases. J. Struct. Biol. 146, 11–31 (2004). An evolutionary classification of the vast class of the cellular and viral ATPases in the context of the origins of primordial genetic systems, last universal common ancestor, bacteria, archaea and eukaryotes. It describes S3Hs as a distinct branch within the AAA+ class of ATPases.
Maaty, W. S. et al. Characterization of the archaeal thermophile Sulfolobus turreted icosahedral virus validates an evolutionary link among double-stranded DNA viruses from all domains of life. J. Virol. 80, 7625–7635 (2006).
Benson, S. D., Bamford, J. K., Bamford, D. H. & Burnett, R. M. Does common architecture reveal a viral lineage spanning all three domains of life? Mol. Cell 16, 673–685 (2004).
Dolja, V. V., Boyko, V. P., Agranovsky, A. A. & Koonin, E. V. Phylogeny of capsid proteins of rod-shaped and filamentous plant viruses: two families with distinct patterns of sequence and probably structure conservation. Virology 184, 79–86 (1991).
McGeoch, D. J., Rixon, F. J. & Davison, A. J. Topics in herpesvirus genomics and evolution. Virus Res. 117, 90–104 (2006).
Dolja, V. V. & Koonin, E. V. Phylogeny of capsid proteins of small icosahedral RNA plant viruses. J. Gen. Virol. 72 1481–1486 (1991).
Schneemann, A., Reddy, V. & Johnson, J. E. The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv. Virus Res. 50, 381–466 (1998).
Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797 (2004).
Jobb, G., von Haeseler, A. & Strimmer, K. TREEFINDER: a powerful graphical analysis environment for molecular phylogenetics. BMC Evol. Biol. 4, 18 (2004).
Whelan, S. & Goldman, N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 18, 691–699 (2001).
Ronquist, F. & Huelsenbeck, J. P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574 (2003).
Acknowledgements
This paper is dedicated to Professor Vadim I. Agol. We thank V. Agol and T. Senkevich for critical reading of the manuscript and useful comments. E.V.K. and Y.I.W. are supported by the Department of Health and Human Services (National Library of Medicine, National Institutes for Health) intramural research funds. The research in V.V.D.'s laboratory is partially supported by National Institutes for Health grant GM053190 and BARD award no. IS-3,784-05.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary information S1 (Table)
(PDF 180 kb)
Supplementary information S2 (Figure)
(PDF 208 kb)
Supplementary information S3 (Figure)
(PDF 117 kb)
Supplementary information S4 (Figure)
(PDF 116 kb)
Related links
Related links
DATABASES
Entrez Genome
FURTHER INFORMATION
Glossary
- Virosphere
-
Also termed virus world, the virosphere is the entirety of viruses and virus-like agents comprising a genetic pool that is continuous in space and time and encompasses, in particular, hallmark viral genes that encode essential functions of many diverse viruses but are not found in genomes of cellular life forms.
- Superfamily
-
In this context, a superfamily is a large group of viral families that are thought to have evolved from a common ancestor.
- Picornaviruses
-
Narrowly defined, picornaviruses are a family of small, positive-strand RNA viruses that infect animals including humans (for example, poliovirus and foot-and-mouth disease virus). Broadly defined, the superfamily of picorna-like viruses consists of many families of RNA viruses that infect animals, plants and diverse unicellular eukaryotes, and appear to be evolutionarily related to picornaviruses.
- Jelly-roll fold
-
The jelly-roll fold is a characteristic structural fold of the capsid proteins that comprise the icosahedral capsids of a variety of viruses including most of the picorna-like viruses.
- Maximum likelihood
-
Generally, maximum likelihood is the statistical methodology used to fit a mathematical model of a process to the available data. In the context of phylogenetic analysis, maximum-likelihood methods use evolution models of various degrees of complexity to infer probability distributions for all possible topologies of a phylogenetic tree and, accordingly, assign likelihood values to particular topologies.
- Clade
-
A clade is a taxonomic group that consists of a single common ancestor and all its descendants; in a phylogenetic tree, a clade is always either a terminal branch or a compact subtree.
- Horizontal virus transfer
-
(HVT). Cross-species virus transmission and adaptation to a new host.
- Retroelements
-
Diverse genetic elements that encode a reverse transcriptase and, accordingly, replicate through a genetic cycle that includes a step of DNA synthesis on a RNA template.
Rights and permissions
About this article
Cite this article
Koonin, E., Wolf, Y., Nagasaki, K. et al. The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat Rev Microbiol 6, 925–939 (2008). https://doi.org/10.1038/nrmicro2030
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2030
This article is cited by
-
The cryptic microbiota of plant parasitic and entomopathogenic nematodes: diversity, effects on host biology and potential in plant protection
Journal of Pest Science (2024)
-
RNA virus discoveries in the electric ant, Wasmannia auropunctata
Virus Genes (2023)
-
Major Transitions as Groupoid Symmetry-Breaking in Nonergodic Prebiotic, Biological and Social Information Systems
Acta Biotheoretica (2022)
-
Re-examination of nepovirus polyprotein cleavage sites highlights the diverse specificities and evolutionary relationships of nepovirus 3C-like proteases
Archives of Virology (2022)
-
A second capsidless hadakavirus strain with 10 positive-sense single-stranded RNA genomic segments from Fusarium nygamai
Archives of Virology (2021)