Abstract
Multiple sclerosis (MS) is a chronic autoimmune disorder with a complex etiology, and Epstein–Barr virus (EBV) is considered the leading cause. While understanding the role of EBV infection in the pathogenesis of MS in human subjects is crucial, animal models, particularly nonhuman primates (NHPs), would provide an ideal controlled environment for testing EBV hypotheses and identifying potential therapeutic targets. Here in this Review we address clinically relevant questions regarding the link between EBV infection and MS to inform the development and refinement of virally induced NHP models. We focus on integrating known EBV-related risk factors for MS, including age at infection, infectious mononucleosis, genetic predispositions such as the human leukocyte antigen (HLA)-DR15 haplotype, sex-specific susceptibility, low vitamin D levels and CD8+ T cell deficiency. We also explore the application of these risk factors in model development, investigate why most EBV-infected individuals do not develop MS and propose potential disease-modifying therapeutic options and vaccines. Integrating these approaches into NHP models will improve our understanding of MS pathogenesis and guide the development of targeted strategies for disease management and prevention. We propose to develop a refined EBV infection NHP model of MS coupled with CD8+ cell depletion and other inclusion and exclusion criteria.

Similar content being viewed by others
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease characterized by inflammation, demyelination and varying degrees of neuroaxonal loss in the central nervous system (CNS)1. Demyelination results in a broad spectrum of neurological symptoms, including motor and sensory deficits and cognitive and visual impairment. The development of MS is complex and seems to involve a combination of environmental, genetic and immunological factors2. Environmental factors, such as a Western diet, vitamin D deficiency, smoking and disrupted microbiota, may interact with certain genetic variations linked to MS, resulting in dysregulated immune responses3. A recent epidemiological study found a correlation between infection with the Epstein–Barr virus (EBV) and an increased susceptibility to developing MS4. EBV, a ubiquitous human herpesvirus, is known to establish lifelong latent infection in B lymphocytes and has been implicated in numerous diseases, including MS. Although the exact etiology of MS remains elusive, accumulating evidence suggests that EBV infection plays a pivotal role in its pathogenesis3. Almost all patients with MS test positive for EBV antibodies, and those who experience symptomatic primary EBV infections, such as infectious mononucleosis, are at a considerably higher risk of developing MS later in life3. The relationship between EBV and MS is complex and influenced by several factors, including the timing of EBV infection, genetic predisposition and the immune response. For instance, the risk of developing MS is notably higher when EBV infection occurs during adolescence or adulthood compared with early childhood2,5. Sex differences, genetic factors such as specific human leukocyte antigen (HLA) haplotypes (for example, HLA-DR15) and environmental factors (for example, vitamin D levels) also influence susceptibility to MS. Despite this knowledge, the mechanisms through which EBV contributes to MS remain poorly understood, particularly in the context of age and CD8+ T cell function. Nonhuman primate (NHP) models offer a unique opportunity to bridge this knowledge gap6. NHPs, particularly rhesus macaques, are valuable for studying the complex interactions between EBV and MS owing to their physiological and immunological similarities to humans. These models would allow researchers to investigate the pathophysiological processes underlying EBV-associated MS and to test therapeutic interventions in a controlled environment7. However, current NHP models of MS are limited in their ability to fully recapitulate the disease’s complexity and determine how it relates to EBV. This Review aims to (1) explore EBV-related risk factors for MS, such as the timing of infection, genetic predisposition (for example, HLA-DRB1*15:01), environmental influences (for example, vitamin D levels) and CD8+ T cell response; and (2) to develop and refine virus-induced NHP models of MS. This Review also provides a comprehensive overview of how NHP models can be optimized to explore the intricate relationship between EBV and MS, offering new avenues for therapeutic interventions and improving our understanding of MS pathogenesis in the context of EBV infection.
EBV-related risk factors for MS
Understanding the risk factors that link EBV to MS is crucial to unraveling the complex relationship between viral infections and autoimmune diseases. EBV, a ubiquitous human herpesvirus, is strongly associated with MS, yet not all individuals infected with EBV develop the disease3. Identifying the risk factors that affect this relationship can help to refine our understanding of MS pathogenesis and inform the development of targeted preventive and therapeutic strategies. In this section, we will focus on primary EBV-related risk factors that contribute to the progression of MS, including infectious mononucleosis, incubation period, genetic factors, sex differences, vitamin D deficiency and CD8+ T cell deficiency.
Infectious mononucleosis
One of the key EBV-related risk factors for MS is infectious mononucleosis, a common consequence of EBV infection8. Infectious mononucleosis is characterized by fever, sore throat, lymphadenopathy and fatigue9. It usually arises during primary EBV infection, typically in adolescents or young adults10. Mononucleosis and MS are more likely to occur when primary EBV infection occurs after age 10, a period characterized by a reduction in thymic negative selection of autoreactive T cells and a peak in T helper 1 (Th1) cell-mediated immune responses3. The association between infectious mononucleosis and an increased risk of developing MS has been well documented in prospective studies11: The risk of developing MS in individuals with EBV infection is 32-fold higher compared with uninfected individuals. This risk is even higher when EBV infection leads to symptomatic-to-severe infectious mononucleosis, particularly for those carrying the HLA-DR15 haplotype12. Severe infectious mononucleosis might exacerbate immune dysregulation and contribute to the autoimmune response observed in MS13. The mechanisms underlying this increased risk may involve higher and more prolonged viral loads and immune activation during acute EBV infection, leading to a cascade of inflammatory events that predispose individuals to autoimmune diseases3. EBV also creates an extralymphatic viral reservoir in the CNS during infectious mononucleosis. During this period, approximately 50% of memory B cells may test positive for EBV14. In summary, infectious mononucleosis is a key factor in EBV-induced MS and should be carefully considered in NHP model development. Refining these models will improve our understanding of MS and inform targeted prevention and treatment strategies. Integrating these insights into research and clinical practice will improve MS management and patient outcomes.
Age of infection
Age at initial EBV infection also plays a vital role in the pathogenesis of MS. Primary EBV infection occurring before 5 years of age is frequently asymptomatic, whereas infection during adolescence can lead to infectious mononucleosis, which causes a substantial expansion of atypical CD8+ T cells and NK cells. Most individuals maintain effective long-term immune control over the virus with sporadic, rapidly suppressed reactivation events3. The majority of children in developing countries acquire asymptomatic EBV infection before age 3 and develop antibodies against EBV by age 10 (ref. 15). Early life exposure to microbes including EBV due to unsanitary conditions and poor hygiene in developing countries may contribute to protection against autoimmunity16. In developed countries, around 50% of children lack antibodies against EBV by age 10. Of these, many acquire EBV infections orally during their teenage years or early adulthood10, around half of which present as acute infectious mononucleosis17. These delayed initial infections may explain the increased risk of MS in individuals with a history of infectious mononucleosis8: The risk of developing MS is markedly higher if EBV infection occurs during adolescence or early adulthood compared with early childhood4. This differential risk may be related to the developmental state of the immune system at the time of infection and the potential for critical immunological disturbances during later stages of life18. NHP models of MS must carefully consider the timing of EBV infection for accurate recapitulation of human disease.
Major histocompatibility complex haplotypes
Genetic predisposition plays a crucial role in susceptibility to MS. One of the most well-established genetic risk factors for MS is the presence of a specific major histocompatibility complex (MHC), the HLA-DR15 haplotype19, which interacts with EBV infection to confer a threefold or higher risk of developing MS20. HLA-DR15 alleles, including HLA-DRB1*15:01, are known to mediate EBV entry into B cells, raising the possibility that the pathways facilitating viral entry could also play a role in increasing MS risk3. The HLA-DRB1*15:01 allele is thought to influence the immune response to EBV, possibly by increasing autoimmune reaction against myelin. In humanized mouse models, the MHC class II molecule HLA-DRB1*15:01 is associated with less effective control of EBV infection12, and CD4+ T cells activated during EBV infection recognize EBV-transformed B cells in an HLA-DRB1*15:01-restricted manner, exhibiting a higher propensity for cross-reactivity with myelin autoantigens. This cross-reactivity is believed to contribute to the autoimmune processes underlying MS21. HLA-DR15 synergistically promotes the activation of hyperreactive T cell compartments that show reduced efficiency in controlling viral infections and harbor cross-reactive CD4+ T cell clones12. Patients with MS with HLA-DRB1*15 or HLA-B*07 alleles have elevated EBV viral loads, while those with HLA-A*02 have lower viral loads22, suggesting that both class I and class II MHC molecules may influence EBV latency control. These associations highlight the role of CD8+ T cells, restricted by MHC class I molecules such as HLA-A*02 and HLA-B*07, in differentially mediating antiviral immunity. Effective CD8+ T cell responses are crucial for controlling EBV-infected B cells, and impaired CD8+ T cell surveillance may contribute to the persistence of EBV and the increased risk of MS. MS-associated risk alleles are linked with altered gene expression patterns, and elevated expression of these HLA alleles can influence peptide selection and presentation, which may contribute to peptide mimicry and autoreactivity23. Understanding the interplay between genetic predisposition and EBV infection is crucial for MS research. Studying MHC haplotypes and other risk genes in NHP models, including genetic modifications such as CRISPR–Cas9, can refine MS models and uncover disease mechanisms.
Sex differences
Sex differences are a well-recognized factor in MS epidemiology and risk24. Women are more likely to experience symptomatic EBV infections such as infectious mononucleosis and are subsequently at a higher risk for developing MS compared with men. A study of 2,487 Danish patients with infectious mononucleosis reported that the cumulative risk of developing infectious mononucleosis before age 30 was 13.3% for males and 22.4% for females25. A single-center retrospective study found that male patients generally experienced EBV-induced infectious mononucleosis later in life than women, and these infections were more likely to cause headache and leukocytosis26. Hormonal differences, immune system variations, oral and gut microbiota, and genetic factors may contribute to this increased susceptibility in women27. Estrogen, while known to modulate immune responses—by regulating transcription factor FoxP3, programmed cell death protein 1, interferon gamma (IFN-γ) promoter region, T-bet and cytotoxic T-lymphocyte antigen 4—exerts complex and sometimes opposing effects28. The role of estrogen in autoimmunity is highly context dependent29. While it can enhance immune activity and contribute to increased susceptibility to autoimmune diseases, it may also exert anti-inflammatory effects in conditions like MS. Notably, MS symptoms often improve during pregnancy, when a shift toward a Th2-dominant immune profile (not Th1) is thought to reduce inflammation and disease activity30. The sex differences in MS may also reflect the earlier onset of puberty in females compared with males, and the tendency in heterosexual relationships for the female partner to be younger than the male partner25. Females typically have higher numbers of CD4+ T cells and a higher CD4+/CD8+ T cell ratio compared with age-matched males, reflecting a relatively lower proportion of CD8+ T cells within the overall T cell compartment31. As CD8+ T cells are critical for controlling viral infections such as EBV, this lower relative abundance may impair effective viral clearance, potentially contributing to an increased susceptibility to EBV-related conditions such as infectious mononucleosis and MS. Hormonal factors, combined with this immune imbalance, probably contribute to the increased susceptibility of females to MS, where an overactive immune system attacks the nervous system. Incorporating sex-specific differences in EBV infection and MS risk is vital for developing accurate NHP models. Including both sexes or focusing on female animals can clarify how these factors influence disease progression and inform targeted prevention and treatment strategies.
Vitamin D levels
Vitamin D is recognized for its immunomodulatory properties, particularly its influence on T cell function and the regulation of inflammation32. Low levels of vitamin D are associated with an increased risk of MS, probably due to its role in modulating immune system function and influencing the immune response to EBV33. The proposed mechanism is that vitamin D increases the availability of CD8+ T cells capable of targeting and controlling EBV infection. The vitamin D receptor is expressed at high levels on activated CD8+ T cells, suggesting an important role for vitamin D in modulating this activity of CD8+ T cells34. Vitamin D also enhances the mitogen-induced proliferation of CD8+ T cells and lowers the CD4/CD8 ratio in bovine peripheral blood mononuclear cells35. Supplemental vitamin D has been associated with increased CD8+ T cell count, while vitamin D deficiency correlates with a reduced proportion of CD8+ T cells and an elevated CD4/CD8 ratio36.
Vitamin D deficiency plays a role in the development of MS by contributing to the depletion of CD8+ T cells, which are crucial for controlling EBV infection. Because vitamin D metabolism in NHPs closely resembles that in humans, including hydroxylation pathways (that is, 25-hydroxylation and 1α-hydroxylation) and immunomodulatory effects, NHP models provide a relevant and translationally valuable system for studying the immune consequences of vitamin D deficiency37. This hypothesis can be tested in NHP models by examining how vitamin D deficiency impacts CD8+ T cell responses, EBV load and MS-like symptoms. Studies using dietary supplementation can clarify the role of vitamin D in immune regulation and EBV control, offering insights into MS pathogenesis and potential therapeutic strategies. Research could also include controlled sunlight exposure to evaluate its effects on CD8+ T cell counts, EBV load and clinical symptoms associated with MS.
CD8+ T cell deficiency, EBV control and MS
CD8+ T cells play a key role in immune defense against EBV by recognizing viral peptides via TCRs on MHC class I molecules38. Upon activation, they induce apoptosis in infected cells through perforin and granzymes while releasing IFN-γ to enhance antiviral immunity and suppress viral replication39. A pilot study of ten patients with MS also found elevated levels of interleukin (IL)-4 and IFN-γ mRNA in highly differentiated CD8+ T cells40. CD8+ T cells recognize EBV antigens (for example, EBNA3A, EBNA3B and EBNA3C) essential for the virus’ ability to persist and proliferate41. CD8+ T lymphocytes specific for the EBV-encoded nuclear antigen 1 (EBNA1)-derived epitope HPVGEADYFEY were also found in the blood and cerebrospinal fluid (CSF) of patients with MS42. There was a correlation between antibodies specific to EBNA1 and CD8+ T cells that target EBV in individuals with a first neurological episode of MS (called clinically isolated syndrome) and relapsing–remitting MS43. Patients with relapsing–remitting MS have high populations of CD8bright cells that specifically recognize the HLA-E–BZLF1 complex. The frequency of CD8+ T lymphocytes responding to specific EBV-derived epitopes, including EBNA3A (HLA-A2–CLG) and LMP-2 (HLA-B7–RPP), is higher in patients with MS compared with healthy controls44. In EBV infection, CD8+ T cells selectively target and eliminate B cells that are infected with EBV17. Evidence suggests that multifunctional CD8+CD57+ cytotoxic T cells in healthy donors could eliminate EBV-infected cells45. An analysis of postmortem brain samples from 12 donors with progressive MS and known HLA class I genotype revealed the presence of cytotoxic CD8+ T cells that specifically target EBV in the CNS. These CD8+ T cells were shown to be positive for CD107a, indicating cytotoxic characteristics, and colocalized to EBV-infected cells41. The presence of the EBV lytic protein BZLF1 and the interaction between cytotoxic CD8+ T lymphocytes and EBV-infected plasma cells in inflammatory areas in the meninges and white matter have been observed in untreated patients with MS46. In individuals with MS, T cell receptor-β sequences of CD8+ T cells that react to EBV, including various commonly shared EBV-specific sequences, were shown to be enriched intrathecally.
CD8+ T cell deficiency may contribute to the accumulation of EBV-infected B cells in the MS brain41,47,48. CNS-recruited CD8+ T cells typically provide immune surveillance, but in MS, their reduced frequency and impaired function hinder their ability to control EBV48,49,50,51. Patients with MS, particularly in advanced stages, show deficits in effector memory CD8+ T cells and decreased expression of EOMES and T-bet, key regulators of cytotoxic immunity47,52,53,54. Increased PD-1 expression on CD8+ T cells correlates with higher viral loads, suggesting a potential mechanism of EBV-driven MS pathogenesis through CD8+ T cell exhaustion55. A minority of patients may experience reduced HLA class II expression on B cells, which can prevent the CD8+ T cell response to EBV by diminishing CD4+ T cell help56. However, reports indicate higher frequencies of CD8+ T cells that produce IL-4 and IL-10, and higher frequencies of T-bet+ CD4 and CD8 T cells in primary progressive disease57,58. Patients with clinically isolated syndrome and those with active MS exhibit a higher percentage of CD8+ T cells expressing activation markers CD26 and CD69 compared with patients with inactive disease or healthy individuals59. This increased CD8+ T cell activity may serve as a compensatory mechanism to control MS progression. IFN-β treatment regulates CD8+ T cell activation, potentially influencing disease activity from onset. After IFN-β treatment, the percentage of CD8+ T cells expressing IL-10 and IL-13 increases, while those positive for CD26 and CD71 decrease59.
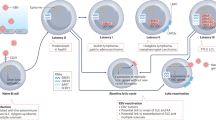
The total number of CD8+ T cells in healthy individuals drops threefold between the ages of 2 and 16 (ref. 60). If primary EBV infection occurs during adolescence or adulthood, after the natural decline in CD8+ T cells, the same genetic deficiency is more likely to impair EBV infection control17, possibly explaining the higher prevalence of MS when the initial infection occurs after puberty61. However, a genetically determined CD8+ T cell deficiency does not affect the ability to control EBV infection when it occurs in early childhood, unless the deficiency is severe. Aging is related to a decline in immune function, a phenomenon known as immunosenescence, which can affect various immune cells, including CD8+ T cells62 (Fig. 1). In elderly individuals, CD8+ T cells exhibit impaired cytotoxic function, reduced proliferative capacity and altered cytokine production63. These changes can impair the ability to control viral infections, including EBV, which leads to the progression of MS.
In people with a severe genetic CD8+ T cell deficiency, autoimmune disorders such as MS appear earlier in life and progress more quickly. Vitamin D and sunlight deprivation at higher latitudes exacerbate genetic CD8+ T cell depletion, elevating the risk and development of MS. Adapted from ref. 17, license: https://creativecommons.org/licenses/by/3.0/, and modified with BioRender.com.
A case–control study of 64 Australian patients with MS and 68 matched healthy controls found CD8+ T cell deficiency in MS, with a reduced response to EBV-infected B cells that worsened with age64. This deficiency included fewer lymphoblastoid cell line-specific CD8+ T cells, especially in secondary progressive MS, suggesting T cell exhaustion. The age-related decline in CD8+ T cells in MS may stem from the depletion of EBV-specific T cells due to prolonged immune activation64. The progressive decline of CD8+ T cells due to accelerated aging, along with the resulting increase in EBV burden, may be responsible for the age-related accumulation of disability in MS65.
Immunosenescence leads to reduced immune cell production, altered cytokine profiles and impaired function62. CD8+ T cells are particularly affected by aging, including a decline in naive CD8+ T cells, diminished proliferative capacity, impaired cytotoxic function and telomere shortening, all of which weaken the response to EBV infection63,66,67,68,69. Aging significantly affects CD8+ T cell function, with profound implications for viral control and the pathogenesis of MS. The decline in CD8+ T cell responses in aging due to thymic involution, telomere shortening and altered signaling pathways (PI3K–AKT, mTOR, NF-κB, JAK–STAT and cytokine signaling pathways) may contribute to reduced control of EBV and increased autoimmunity70,71,72,73.
In summary, CD8+ T cells play a key role in MS, especially in controlling EBV infection. Using age-specific NHP models or inducing CD8+ T cell depletion can help to uncover their role in MS progression. These models are essential for developing targeted therapies to modulate CD8+ T cell responses and manage EBV-driven MS.
Limitations of current animal models of MS
The experimental autoimmune encephalomyelitis (EAE) model is the most frequently used animal model in MS research, and its historical and scientific importance is well established. Rivers et al. first described it in monkeys in 1933 (refs. 74,75,76). In the 1940s and 1950s, EAE models were developed in mice and rats and established as fundamental tools in MS research75. The mouse EAE model has dramatically advanced our understanding of the autoimmune mechanisms and demyelination processes in MS, and its utility extends to mechanistic studies, as it can be induced in various genetically modified mouse strains77. The widespread application of this model in MS research is largely due to its amenability to genetic manipulation, capacity for large-scale experimentation and cost-effectiveness77.
The mouse EAE model has limitations in replicating the full complexity of human MS, particularly in lesion localization and immune response. While human MS pathology suggests a key role for CD8+ T cells and B cells, CD4+ T cells dominate in EAE78. EAE follows a rapid disease course, whereas human MS often presents with a relapsing–remitting pattern. Thus, while valuable for studying immune mechanisms, the mouse model does not fully reflect the clinical progression of MS79. To overcome these limitations, NHP models, particularly the marmoset EAE model, have been developed. The marmoset EAE model exhibits pathological features that more closely resemble human MS and, with a brain structure and immune system more analogous to that of humans, offers greater relevance for studying the intricate pathophysiology of MS75. We found changes in CD8+ T cell and B cell subpopulations consistent with human MS findings in our studies using the marmoset EAE model; however, marmoset EAE lesions were confined to the spinal cord, with no brain involvement (unpublished data).
Current NHP models to study MS
NHP models are pivotal in elucidating the complex interactions between EBV and MS and revealing the immunological mechanisms and viral influences that may contribute to MS pathogenesis6. NHPs allow a deeper understanding of how EBV influences the risk of developing MS (Table 1) and provide valuable insights into immunological and pathological processes that are difficult to investigate in humans. For example, a rhesus lymphocryptovirus (LCV) model successfully replicated important characteristics of human EBV infection, (transmission through the mouth, CD23+ activation, response to EBV antigens and so on)80. Monkeys infected with simian immunodeficiency virus develop B cell lymphomas associated with the Epstein–Barr-like cynomolgus B-lymphotropic herpesvirus, which are similar to EBV-associated B cell lymphomas observed in individuals with acquired immunodeficiency syndrome or organ transplants81.
Several key studies have used NHP models to investigate the pathogenesis of MS and the role of EBV or EBV-like viruses in this process. Haanstra et al. utilized rhesus macaques (Macaca mulatta) to study the effects of herpesvirus papio in combination with an encephalitogenic self-peptide (MOG34–56) and a capsid protein of cytomegalovirus (CMVmcp981–1003). Their findings indicated an increase in CD3+CD8+ T cells, CD8+CD56+ T cells and CD3−CD56+ NK cells, alongside an increase in perivascular inflammatory lesions composed of CD3+ and CD68+ cells82. These results suggest that EBV-induced B-lymphoblastoid cells can cause encephalitis, highlighting a potential mechanism by which EBV may contribute to MS pathology. Jagessar et al. examined LCV infection in NHP B cells and found increased expression of CD70, CD80, proteasome maturation protein, immunoproteasome subunits and LC3-II+ cytosolic structures resembling autophagosomes, along with altered cathepsin expression83. These changes suggest that B cells infected with EBV-related LCV could be linked to MS, highlighting the virus’s potential role in altering immune responses. Anwar Jagessar et al. used marmoset monkeys in an EAE model induced with rhMOG/CFA or MOG34–56/IFA. Treatments included anti-CD20 monoclonal antibodies (mAb) and antibodies targeting BLyS and APRIL. CalHV3 is a type of γ-herpesvirus found in marmosets. It has numerous functional similarities with EBV, such as the ability to transform B cells84. The anti-CD20 mAb reduced the load of CalHV3 DNA in lymphoid organs, while the MOG34–56 group showed increased anti-MOG34–56 T cells and meningeal inflammation85. This study suggests that a herpesvirus-transformed subset of CD20+ B cells may be relevant to the EAE model, providing a potential link to MS. Axthelm et al. studied Japanese macaques (Macaca fuscata) with a spontaneously occurring MS-like disease called Japanese macaque encephalomyelitis (JME). They isolated a novel gamma-herpesvirus in acute JME white matter lesions and plaque-like demyelinated lesions86. Their results support an association between MS and a novel simian herpesvirus, reinforcing the hypothesis that viral infections may play a role in MS pathogenesis. Govindan et al. further explored JME in Japanese macaques, noting decreased CD8+ T cell responses, increased CD4 Th1 and Th17 responses, and increased CD3+ T cells87. They also identified myelin antigens, suggesting that JME could provide new insights into the pathogenesis of inflammatory demyelinating diseases linked to gamma-herpesvirus infection. Estep et al. identified Japanese macaque rhadinovirus (JMRV17792), whose genome is similar to other gamma-herpesviruses88. They isolated JMRV from a Japanese macaque with inflammatory demyelinating encephalomyelitis, akin to MS in humans. This finding underscores the potential of using NHP models to understand the viral contributions to MS.
While NHP models have advanced our understanding of EBV and MS, the role of CD8+ T cell deficiency, particularly with aging, remains underexplored. Current studies focus on broader immunopathological mechanisms, but the impact of age-related CD8+ T cell decline on MS progression is unclear8. Aging reduces CD8+ T cell numbers and function, impairing EBV control and potentially exacerbating MS, but no studies have examined CD8+ T cell deficiency in aged NHP models (Table 1). Longitudinal studies tracking CD8+ T cells, EBV load and MS-like symptoms in aging NHPs are needed to clarify this relationship and explore therapeutic strategies to enhance CD8+ T cell function in older patients.
CMV and its potential protective effect in MS
While EBV is linked to MS pathogenesis, cytomegalovirus (CMV) may play a protective role by enhancing CD8+ T cell responses. CMV infection increases CMV-specific CD8+ T cells, which may also bolster immunity against EBV, potentially reducing its reactivation and mitigating MS-related pathology. A study by Bjornevik et al. (2021) showed that CMV infection enhances the overall repertoire of CD8+ T cells that are cross-reactive to EBV, which may contribute to the suppression of EBV-driven immune dysregulation in MS4. These findings imply that CMV infection could potentially mitigate the autoimmune processes associated with MS. In addition, CMV infection has been shown to lead to an expansion of the CMV-specific CD8+ T cell pool, which might be beneficial in maintaining immune balance. This cross-reactivity between CMV and EBV-specific CD8+ T cells could reduce EBV-induced pathology, acting as a protective mechanism in patients with MS89.
Considerations for developing novel EBV-induced NHP models of MS
NHP models offer great advantages for studying the role of EBV in MS due to their close physiological and immunological resemblance to humans. To develop and refine these models effectively, it is crucial to incorporate specific risk factors known to influence EBV infection and MS susceptibility. This section outlines strategies for creating robust NHP models to elucidate the contribution of EBV to the pathogenesis of MS development.
CD8+ T cell depletion
CD8+ T cells are critical for controlling viral infections, including EBV44, but the specific effects of CD8+ T cell depletion on EBV infection and MS pathogenesis in NHP models are not well explored. Experimental depletion of CD8+ T cells in NHP models could help to elucidate their role in controlling EBV infection by assessing how the absence of CD8+ T cells affects viral load, persistence and reactivation. Previous data suggest that CD8+ T cell depletion leads to increased viral replication and dissemination, highlighting their importance in maintaining viral latency64. The role of CD8+ T cells in preventing autoimmune responses in the context of EBV infection is critical. Depletion studies could investigate whether the absence of CD8+ T cells exacerbates neuroinflammation and demyelination, potentially mimicking MS-like pathology. Understanding how CD8+ T cells modulate immune responses to myelin antigens in the presence of EBV-infected B cells is crucial. While NHP models have been instrumental in revealing the intricate relationship between EBV and MS, the role of CD8+ T cell deficiency in this dynamic, particularly in the context of aging, remains underinvestigated. Filling this research gap will allow a comprehensive understanding of MS and inform effective therapeutic strategies that address both viral control and age-related immune decline.
Selecting nonprotective Mamu haplotypes
In rhesus macaques, the Mamu MHC class I and II alleles exhibit functional similarities to human HLA molecules, making them an important factor in the development of EBV-induced MS models. Specific Mamu haplotypes influence the immune response to viral infections, particularly the ability of CD8+ T cells to recognize and eliminate EBV-infected cells, which is a key determinant in controlling viral persistence and immune dysregulation. Identifying and selecting NHPs with Mamu haplotypes that mirror MS-associated HLA risk alleles is essential for model optimization. Previous studies have shown that HLA-A*02 and HLA-B*07 are associated with differential immune responses to viral infections, including EBV and CD8 response90,91. Given that HLA class II alleles are critical for antigen presentation and shaping adaptive immune responses, targeting Mamu haplotypes analogous to MS-associated HLA variants would help to establish a more translationally relevant model of EBV-induced MS. Future studies should genotype NHPs for Mamu haplotypes before infection to ensure appropriate selection. Integrating transcriptomic and proteomic analyses can further elucidate genetic influences on EBV-induced neuroinflammation. Refining NHP models based on Mamu haplotypes will improve reproducibility and translational relevance, aiding the development of targeted immunotherapies for MS.
Vitamin D deficiency
The risk of MS has been linked to low vitamin D levels before the onset of the disease, and EBV infection is considered a necessary factor for MS development92. Vitamin D receptors are present on EBV-infected B cells, antigen-presenting cells and activated lymphocytes. The bioactive metabolite of vitamin D, dihydroxyvitamin D3, suppresses antibody production and T cell proliferation and promotes a shift in T cells toward a less harmful Th2 phenotype93. It has been suggested that reduced sunlight and vitamin D levels at higher latitudes may contribute to the development of autoimmune diseases by exacerbating CD8+ T cell deficiency, which, in turn, impairs the control of EBV94. Considering the role of vitamin D in regulating immune responses and controlling EBV infection, inducing vitamin D deficiency in NHP models can offer insights into its relationship with MS17. Vitamin D deficiency in NHPs can be induced with a diet devoid of vitamin D2 and D3 but with adequate levels of other nutrients to prevent confounding deficiencies. Regular monitoring of serum 25(OH)D levels and immune markers, including CD8+ T cell counts and function, ensures controlled vitamin D deficiency, allowing studies on its impact on EBV control, immune dysregulation and MS development.
Selecting female monkeys
Women are approximately three times more likely than men to develop MS95. Given the established sex-specific differences, focusing on female NHPs in model development is crucial. Although NHP models can serve as a critical link between rodent EAE models and human patients with MS96,97, female primates would be more frequently afflicted with MS, and using female models can help to reveal sex-specific mechanisms of disease onset and progression. By studying these models, future studies can gain insights into how hormonal and genetic factors interact with EBV to influence MS development.
Selecting susceptible ages of NHPs
Age at EBV infection is crucial for understanding its association with MS. In humans, the strongest correlation between EBV and MS is observed among individuals infected between the ages of 14 and 20 years. Most MS diagnoses occur at age 20–50, often many years after initial EBV exposure3. To accurately translate this in rhesus macaques, it would be pertinent to focus on young adult females aged 4–6 years. An unpublished study noted that no demyelinating lesions were found in rhesus macaques infected with LCV, suggesting that the absence of lesions could be due to the fact that the subjects were predominantly males and infants or juveniles, who are too young to exhibit such lesions.
Immunotherapies in MS
EBV-targeted immunotherapies are being explored to reverse exhausted EBV-targeted T cell responses, particularly the CD8+ cytotoxic T lymphocyte (CTL) response, which is thought to lead to poor virus control and increased MS disease activity54,98. For example, the adoptive transfer of ex-vivo-expanded autologous EBV-specific CD8+ T cells is directed against viral latent proteins98. In an open-label trial of patients with progressive MS, escalating doses of ex-vivo-expanded autologous EBV-reactive T cells targeting EBNA1, LMP1 and LMP2A were administered, and seven out of ten showed improvement99. This approach was subsequently explored using partially MHC-matched allogenic EBV-specific T cells (clinicaltrials.gov NCT03283826)100; however, the phase 2 exploratory trial was negative101. Whether this trial failed because of the poor survival of these allogeneic cells is unclear102. Another possible explanation for the results is inadequate trial design, which included patients with MS with high levels of disability102. Another approach being explored is therapeutic EBV vaccination to boost T cell responses to EBV103, ideally in NHP models of MS.
Conclusion
The relationship between EBV and MS represents a complex interaction of viral, genetic and environmental factors. This Review highlights the critical need for developing and refining NHP models to address clinically relevant questions about the role of EBV in MS pathogenesis. By focusing on key EBV-related risk factors, such as infectious mononucleosis, timing of infection, genetic susceptibility (HLA haplotypes), sex-specific differences, vitamin D levels and CD8+ T cell deficiency, we can better understand how EBV may trigger or drive MS. The proposed NHP-EBV infection models, especially those incorporating CD8 depletion and other relevant factors, offer a promising approach to determining whether EBV acts as a trigger for MS or a driver of its progression. Such models will not only facilitate the evaluation of these theories but also help to reveal why most EBV-infected individuals do not develop MS. These insights could inform the development of disease-modifying therapies, including EBV-targeted immunotherapies and preventive vaccines. By bridging gaps in our understanding of MS and EBV, we can translate research into effective strategies for managing and preventing MS. The integration of these models into clinical research could lead to major advancements in treatment and prevention, ultimately improving outcomes for individuals at risk of or living with MS (Boxes 1 and 2).
References
Tafti, D., Ehsan, M. & Xixis, K. L. Multiple Sclerosis (StatPearls, 2024).
Bjornevik, K., Münz, C., Cohen, J. I. & Ascherio, A. Epstein–Barr virus as a leading cause of multiple sclerosis: mechanisms and implications. Nat. Rev. Neurol. 19, 160–171 (2023).
Soldan, S. S. & Lieberman, P. M. Epstein–Barr virus and multiple sclerosis. Nat. Rev. Microbiol. 21, 51–64 (2023).
Bjornevik, K. et al. Longitudinal analysis reveals high prevalence of Epstein–Barr virus associated with multiple sclerosis. Science 375, 296–301 (2022).
Thomas, O. G., Rickinson, A. & Palendira, U. Epstein–Barr virus and multiple sclerosis: moving from questions of association to questions of mechanism. Clin. Transl. Immunol. 12, e1451 (2023).
Estes, J. D., Wong, S. W. & Brenchley, J. M. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 18, 390–404 (2018).
Hassani, A. & Khan, G. What do animal models tell us about the role of EBV in the pathogenesis of multiple sclerosis? Front. Immunol. 13, 1036155 (2022).
Thacker, E. L., Mirzaei, F. & Ascherio, A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann. Neurol. 59, 499–503 (2006).
Dunmire, S. K., Hogquist, K. A. & Balfour, H. H. Infectious mononucleosis. Curr. Top. Microbiol. Immunol. 390, 211–240 (2015).
Minarovits, J. & Niller, H. H. Epstein Barr Virus: Methods and Protocols (Springer, 2017).
Loosen, S. H. et al. Infectious mononucleosis is associated with an increased incidence of multiple sclerosis: results from a cohort study of 32,116 outpatients in Germany. Front. Immunol. 13, 937583 (2022).
Zdimerova, H. et al. Attenuated immune control of Epstein–Barr virus in humanized mice is associated with the multiple sclerosis risk factor HLA-DR15. Eur. J. Immunol. 51, 64–75 (2021).
Nielsen, T. R. et al. Multiple sclerosis after infectious mononucleosis. Arch. Neurol. 64, 72–75 (2007).
Hochberg, D. et al. Acute infection with Epstein–Barr virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J. Virol. 78, 5194–5204 (2004).
Lai, P. K., Mackay-Scollay, E. M. & Alpers, M. P. Epidemiological studies of Epstein–Barr herpesvirus infection in Western Australia. J. Hyg. 74, 329–337 (1975).
Kondrashova, A. et al. Serological evidence of thyroid autoimmunity among schoolchildren in two different socioeconomic environments. J. Clin. Endocrinol. Metab. 93, 729–734 (2008).
Pender, M. P. CD8+ T-cell deficiency, epstein-barr virus infection, vitamin D deficiency, and steps to autoimmunity: a unifying hypothesis. Autoimmune Dis. 2012, 189096 (2012).
Prendergast, A. J., Klenerman, P. & Goulder, P. J. R. The impact of differential antiviral immunity in children and adults. Nat. Rev. Immunol. 12, 636–648 (2012).
International Multiple Sclerosis Genetics Consortium (IMSGC) et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat. Genet. 45, 1353–1360 (2013).
Sawcer, S. et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219 (2011).
Münz, C. Immune control of human γ-herpes infections. Semin. Arthritis Rheum. 64, 152320 (2024).
Agostini, S. et al. HLA alleles modulate EBV viral load in multiple sclerosis. J. Transl. Med. 16, 80 (2018).
Martin, R., Sospedra, M., Eiermann, T. & Olsson, T. Multiple sclerosis: doubling down on MHC. Trends Genet. 37, 784–797 (2021).
Coyle, P. K. What can we learn from sex differences in MS? J. Pers. Med. 11, 1006 (2021).
Rostgaard, K. et al. Primary Epstein–Barr virus infection with and without infectious mononucleosis. PLoS ONE 14, e0226436 (2019).
Sasaki, Y. et al. Sex difference in clinical presentation of patients with infectious mononucleosis caused by Epstein–Barr virus. J. Infect. Chemother. 26, 1181–1185 (2020).
D’Anca, M. et al. Why is multiple sclerosis more frequent in women? Role of the immune system and of oral and gut microbiota. Appl. Sci. 13, 5881 (2023).
Ysrraelit, M. C. & Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 156, 9–22 (2019).
Deems, N. & Leuner, B. Pregnancy, postpartum and parity: resilience and vulnerability in brain health and disease. Front. Neuroendocrinol. 57, 100820 (2020).
Patas, K., Engler, J. B., Friese, M. A. & Gold, S. M. Pregnancy and multiple sclerosis: feto-maternal immune cross talk and its implications for disease activity. J. Reprod. Immunol. 97, 140–146 (2013).
Klein, S. L. & Flanagan, K. L. Sex differences in immune responses. Nat. Rev. Immunol. 16, 626–638 (2016).
Aranow, C. Vitamin D and the immune system. J. Investig. Med. 59, 881–886 (2011).
Downham, C., Visser, E., Vickers, M. & Counsell, C. Season of infectious mononucleosis as a risk factor for multiple sclerosis: a UK primary care case–control study. Mult. Scler. Relat. Disord. 17, 103–106 (2017).
Veldman, C. M., Cantorna, M. T. & DeLuca, H. F. Expression of 1,25-dihydroxyvitamin D3 receptor in the immune system. Arch. Biochem. Biophys. 374, 334–338 (2000).
Nonnecke, B. J., Franklin, S. T., Reinhardt, T. A. & Horst, R. L. In vitro modulation of proliferation and phenotype of resting and mitogen-stimulated bovine mononuclear leukocytes by 1,25-dihydroxyvitamin D3. Vet. Immunol. Immunopathol. 38, 75–89 (1993).
Žofková, I. & Kancheva, R. L. The effect of 1,25(OH)2 vitamin D3 on CD4+/CD8+ subsets of T lymphocytes in postmenopausal women. Life Sci. 61, 147–152 (1997).
Ziegler, T. E. et al. Comparison of vitamin D metabolites in wild and captive baboons. Am. J. Primatol. 80, e22935 (2018).
Chatterjee, B. et al. CD8+ T cells retain protective functions despite sustained inhibitory receptor expression during Epstein–Barr virus infection in vivo. PLoS Pathog. 15, e1007748 (2019).
Nakiboneka, R. et al. Interferon gamma (IFN-γ) negative CD4+ and CD8+ T-cells can produce immune mediators in response to viral antigens. Vaccine 37, 113–122 (2019).
Jaquiéry, E. et al. Cytokine mRNA profile of Epstein–Barr virus-stimulated highly differentiated T cells in multiple sclerosis: a pilot study. J. Neuroimmunol. 225, 167–170 (2010).
Serafini, B., Rosicarelli, B., Veroni, C., Mazzola, G. A. & Aloisi, F. Epstein–Barr virus-specific CD8 T cells selectively infiltrate the brain in multiple sclerosis and interact locally with virus-infected cells: clue for a virus-driven immunopathological mechanism. J. Virol. 93, e00980-19 (2019).
Erdur, H. et al. EBNA1 antigen-specific CD8+ T cells in cerebrospinal fluid of patients with multiple sclerosis. J. Neuroimmunol. 294, 14–17 (2016).
Jilek, S. et al. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain 131, 1712–1721 (2008).
Höllsberg, P., Hansen, H. J. & Haahr, S. Altered CD8+ T cell responses to selected Epstein–Barr virus immunodominant epitopes in patients with multiple sclerosis. Clin. Exp. Immunol. 132, 137–143 (2003).
Cencioni, M. T. et al. Programmed death 1 is highly expressed on CD8+ CD57+ T cells in patients with stable multiple sclerosis and inhibits their cytotoxic response to Epstein–Barr virus. Immunology 152, 660–676 (2017).
Angelini, D. F. et al. Increased CD8+ T cell response to Epstein–Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 9, e1003220 (2013).
Pender, M. P., Csurhes, P. A., Lenarczyk, A., Pfluger, C. M. M. & Burrows, S. R. Decreased T cell reactivity to Epstein–Barr virus infected lymphoblastoid cell lines in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 80, 498–505 (2009).
van Nierop, G. P. et al. Phenotypic and functional characterization of T cells in white matter lesions of multiple sclerosis patients. Acta Neuropathol. 134, 383–401 (2017).
Beltrán, E. et al. Early adaptive immune activation detected in monozygotic twins with prodromal multiple sclerosis. J. Clin. Invest. 129, 4758–4768 (2019).
Machado-Santos, J. et al. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain J. Neurol. 141, 2066–2082 (2018).
Fransen, N. L. et al. Tissue-resident memory T cells invade the brain parenchyma in multiple sclerosis white matter lesions. Brain J. Neurol. 143, 1714–1730 (2020).
Parnell, G. P. et al. The autoimmune disease-associated transcription factors EOMES and TBX21 are dysregulated in multiple sclerosis and define a molecular subtype of disease. Clin. Immunol. 151, 16–24 (2014).
McKay, F. C. et al. The low EOMES/TBX21 molecular phenotype in multiple sclerosis reflects CD56+ cell dysregulation and is affected by immunomodulatory therapies. Clin. Immunol. 163, 96–107 (2016).
Pender, M. P., Csurhes, P. A., Pfluger, C. M. & Burrows, S. R. Deficiency of CD8+ effector memory T cells is an early and persistent feature of multiple sclerosis. Mult. Scler. 20, 1825–1832 (2014).
Najmadini, A. et al. Increased expression of PD-1 in CD8+ CD3+ T cells correlates with EBV viral load in MS patients. J. Neurovirol. 28, 497–504 (2022).
Pender, M. P., Csurhes, P. A., Pfluger, C. M. M. & Burrows, S. R. Decreased CD8+ T cell response to Epstein–Barr virus infected B cells in multiple sclerosis is not due to decreased HLA class I expression on B cells or monocytes. BMC Neurol. 11, 95 (2011).
Killestein, J. et al. Intracellular cytokine profile in T-cell subsets of multiple sclerosis patients: different features in primary progressive disease. Mult. Scler. 7, 145–150 (2001).
Frisullo, G. et al. Type 1 immune response in progressive multiple sclerosis. J. Neuroimmunol. 249, 112–116 (2012).
Jensen, J., Langkilde, A. R., Frederiksen, J. L. & Sellebjerg, F. CD8+ T cell activation correlates with disease activity in clinically isolated syndromes and is regulated by interferon-beta treatment. J. Neuroimmunol. 179, 163–172 (2006).
Comans-Bitter, W. M. et al. Immunophenotyping of blood lymphocytes in childhood. Reference values for lymphocyte subpopulations. J. Pediatr. 130, 388–393 (1997).
Haahr, S., Plesner, A. M., Vestergaard, B. F. & Höllsberg, P. A role of late Epstein–Barr virus infection in multiple sclerosis. Acta Neurol. Scand. 109, 270–275 (2004).
Liu, Z. et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct. Target. Ther. 8, 1–16 (2023).
Nikolich-Zugich, J., Li, G., Uhrlaub, J. L., Renkema, K. R. & Smithey, M. J. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin. Immunol. 24, 356–364 (2012).
Pender, M. P., Csurhes, P. A., Pfluger, C. M. M. & Burrows, S. R. CD8 T cell deficiency impairs control of Epstein–Barr virus and worsens with age in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 83, 353–354 (2012).
Confavreux, C. & Vukusic, S. Age at disability milestones in multiple sclerosis. Brain J. Neurol. 129, 595–605 (2006).
Plunkett, F. J. et al. The flow cytometric analysis of telomere length in antigen-specific CD8+ T cells during acute Epstein–Barr virus infection. Blood 97, 700–707 (2001).
Bellon, M. & Nicot, C. Telomere dynamics in immune senescence and exhaustion triggered by chronic viral infection. Viruses 9, 289 (2017).
Brunner, S., Herndler-Brandstetter, D., Weinberger, B. & Grubeck-Loebenstein, B. Persistent viral infections and immune aging. Ageing Res. Rev. 10, 362–369 (2011).
Lee, K. et al. Characterization of age‐associated exhausted CD8+ T cells defined by increased expression of Tim‐3 and PD‐1. Aging Cell 15, 291–300 (2016).
Gracias, D. T. et al. Phosphatidylinositol 3-kinase p110δ isoform regulates CD8+ T cell responses during acute viral and intracellular bacterial infections. J. Immunol. 196, 1186–1198 (2016).
Pearce, V. Q., Bouabe, H., MacQueen, A. R., Carbonaro, V. & Okkenhaug, K. PI3Kδ regulates the magnitude of CD8+ T cell responses after challenge with Listeria monocytogenes. J. Immunol. 195, 3206–3217 (2015).
Chen, Y. et al. Regulation of CD8+ T memory and exhaustion by the mTOR signals. Cell. Mol. Immunol. 20, 1023–1039 (2023).
Bektas, A., Schurman, S. H., Sen, R. & Ferrucci, L. Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 102, 977–988 (2017).
Rivers, T. M., Sprunt, D. H. & Berry, G. P. Observations on attempts to produce acute disseminated encephalomyelitis in monkeys. J. Exp. Med. 58, 39–52 (1933).
‘t Hart, B. A., van Kooyk, Y., Geurts, J. J. G. & Gran, B. The primate autoimmune encephalomyelitis model; a bridge between mouse and man. Ann. Clin. Transl. Neurol. 2, 581–593 (2015).
Procaccini, C., De Rosa, V., Pucino, V., Formisano, L. & Matarese, G. Animal models of multiple sclerosis. Eur. J. Pharmacol. 759, 182–191 (2015).
Hart, T. et al. The primate EAE model points at EBV-infected B cells as a preferential therapy target in multiple sclerosis. Front. Immunol. 4, 1–10 (2013).
Lassmann, H. & Bradl, M. Multiple sclerosis: experimental models and reality. Acta Neuropathol. 133, 223–244 (2017).
Skundric, D. Experimental models of relapsing-remitting multiple sclerosis: current concepts and perspective. Curr. Neurovasc. Res. 2, 349–362 (2005).
Moghaddam, A. et al. An animal model for acute and persistent Epstein–Barr virus infection. Science 276, 2030–2033 (1997).
Feichtinger, H. et al. A monkey model for Epstein Barr virus-associated lymphomagenesis in human acquired immunodeficiency syndrome. J. Exp. Med. 176, 281–286 (1992).
Haanstra, K. G., Wubben, J. A. M., Jonker, M. & Hart, B. A. ‘t: Induction of encephalitis in rhesus monkeys infused with lymphocryptovirus-infected B-cells presenting MOG34–56 peptide. PLoS ONE 8, e71549 (2013).
Jagessar, S. A. et al. Lymphocryptovirus infection of nonhuman primate b cells converts destructive into productive processing of the pathogenic CD8 T cell epitope in myelin oligodendrocyte glycoprotein. J. Immunol. 197, 1074–1088 (2016).
Cho, Y.-G. et al. An Epstein–Barr-related herpesvirus from marmoset lymphomas. Proc. Natl Acad. Sci. USA 98, 1224–1229 (2001).
Anwar Jagessar, S. et al. The different clinical effects of anti-BLyS, anti-APRIL and anti-CD20 antibodies point at a critical pathogenic role of γ-herpesvirus infected B cells in the marmoset EAE model. J. Neuroimmune Pharmacol. 8, 727–738 (2013).
Axthelm, M. K. et al. Japanese macaque encephalomyelitis: a spontaneous multiple sclerosis-like disease in a nonhuman primate. Ann. Neurol. 70, 362–373 (2011).
Govindan, A. N. et al. Myelin‐specific T cells in animals with Japanese macaque encephalomyelitis. Ann. Clin. Transl. Neurol. 8, 456–470 (2021).
Estep, R. D., Hansen, S. G., Rogers, K. S., Axthelm, M. K. & Wong, S. W. Genomic characterization of Japanese macaque rhadinovirus, a novel herpesvirus isolated from a nonhuman primate with a spontaneous inflammatory demyelinating disease. J. Virol. 87, 512–523 (2013).
van den Berg, S. P. H. et al. Quantification of T-cell dynamics during latent cytomegalovirus infection in humans. PLoS Pathog. 17, e1010152 (2021).
Jones, K. et al. The impact of HLA class I and EBV latency-II antigen-specific CD8+ T cells on the pathogenesis of EBV+ Hodgkin lymphoma. Clin. Exp. Immunol. 183, 206–220 (2016).
Jilek, S. et al. HLA-B7-restricted EBV-specific CD8+ T cells are dysregulated in multiple sclerosis. J. Immunol. 188, 4671–4680 (2012).
Rolf, L. et al. Exploring the effect of vitamin D3 supplementation on the anti-EBV antibody response in relapsing-remitting multiple sclerosis. Mult. Scler. 24, 1280–1287 (2018).
Hewison, M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol. Metab. Clin. North Am. 39, 365–379 (2010).
Holmøy, T. Vitamin D status modulates the immune response to Epstein Barr virus: synergistic effect of risk factors in multiple sclerosis. Med. Hypotheses 70, 66–69 (2008).
Angeloni, B. et al. A case of double standard: sex differences in multiple sclerosis risk factors. Int. J. Mol. Sci. 22, 3696 (2021).
’t Hart, B. A. & Bajramovic, J. J. Non-human primate models of multiple sclerosis. Drug Discov. Today Dis. Models 5, 97–104 (2008).
Ransohoff, R. M. Animal models of multiple sclerosis: the good, the bad and the bottom line. Nat. Neurosci. 15, 1074–1077 (2012).
Pender, M. P. & Burrows, S. R. Epstein–Barr virus and multiple sclerosis: potential opportunities for immunotherapy. Clin. Transl. Immunol. 3, e27 (2014).
Pender, M. P. et al. Epstein–Barr virus-specific T cell therapy for progressive multiple sclerosis. JCI Insight 3, e124714 (2018).
Bar-Or, A. et al. Epstein–Barr virus in multiple sclerosis: theory and emerging immunotherapies. Trends Mol. Med. 26, 296–310 (2020).
Meglio, M. ATA188 fails to meet primary end point in phase 2 EMBOLD study of progressive MS. NeurologyLive https://www.neurologylive.com/view/ata188-fails-meet-primary-end-point-phase-2-embold-study-progressive-ms (2023).
Giovannoni, G., Hawkes, C. H., Lechner-Scott, J., Levy, M. & Yeh, E. A. Emboldened or not: the potential fall-out of a failed anti-EBV trial in multiple sclerosis. Mult. Scler. Relat. Disord. 81, 105364 (2024).
Moderna announces first participant dosed in phase 1 study of its mRNA Epstein–Barr virus (EBV) vaccine. Moderna https://investors.modernatx.com/news/news-details/2022/Moderna-Announces-First-Participant-Dosed-in-Phase-1-Study-of-its-mRNA-Epstein-Barr-Virus-EBV-Vaccine/default.aspx (2022).
Acknowledgements
This work was supported by the NIH P51 Base Grant (P51OD011104) to Tulane National Primate Research Center (RRID: SCR_008167). We thank L. Burton in the Office of Research Proposal Development at Tulane University for her English editing.
Author information
Authors and Affiliations
Contributions
W.-K.K. conceptualized, outlined the work with literature search and revised the manuscript. H.D.N. drafted the initial manuscript and revised the manuscript. All coauthors contributed to writing the manuscript and approved the final version of the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Data sharing statement
Data will be made available on request.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nguyen, H.D., Kim, D., Kim, YH. et al. Epstein–Barr virus and multiple sclerosis: lesson learned to develop better nonhuman primate models. Exp Mol Med (2025). https://doi.org/10.1038/s12276-025-01482-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s12276-025-01482-5