Abstract
Background/Objectives
This study investigated the hitherto unclear association of body mass index (BMI) with age at overall, disability, and disability-free survival in older adults with and without frailty.
Methods
This prospective cohort study enroled 10232 Japanese adults aged ≥65 years, who underwent follow-up for adverse events, from the Kyoto-Kameoka Study conducted from 2011–2016. BMI, calculated based on self-reported height and body weight, was classified into five categories: <18.5, 18.5–21.4, 21.5–24.9, 25.0–27.4, and ≥27.5 kg/m2. Frailty was assessed using the validated Kihon Checklist. The relationships between BMI and disability and mortality were analysed using multivariate Cox proportional hazards models and Laplace regression.
Results
During the 5.3-year median follow-up period (45472 person-years), 2348 (22.9%) incidences of disabilities occurred. After adjusting for confounders, including medical history and lifestyle, individuals in the lowest and highest BMI categories had a higher hazard ratio (HR) of disability [<18.5 kg/m2: HR: 1.31, confidence interval (CI): 1.16–1.49; ≥27.5 kg/m2: HR: 1.27, 95% CI: 1.08–1.49, p for non-linearity <0.001] compared with that of those with BMI = 21.5–24.9 kg/m2. In the 50th percentile differences in age at overall and disability-free survival, participants with BMI < 18.5 kg/m2 were more likely to die before disability incidence [survival with disability (overall survival – disability-free survival): −10.2 months]; those with BMI ≥ 27.5 kg/m2 had longer survival with disability (12.5 months). These relationships were more marked in the frailty-stratified model, where in the BMI ≥ 27.5 kg/m2 group, individuals with frailty survived longer with disability (27.2 months) than did individuals without frailty (6.2 months).
Conclusion
Higher BMI is associated with a longer duration of survival with disability among older adults, especially in those with frailty. Therefore, reversing frailty should be prioritised because individuals with frailty have a shorter probability of disability-free survival than do individuals without frailty, regardless of BMI.
Similar content being viewed by others
Introduction
Frailty, characterised by the loss of integrity and impaired function of multiple physiological systems [1,2,3], is a geriatric syndrome closely associated with mortality [4,5,6,7]. Its prevalence increases with age [4, 5] and is 12–24% in individuals aged ≥50 years [8]. Thus, accumulating evidence on appropriate care for preserving functional independence is required for prolonging lifespan for older adults with frailty [9].
Body mass index (BMI) is a convenient measure for evaluating thinness and fatness. The mean BMI differs among geographical regions, such as America, Europe, and Japan [10]. Furthermore, compared with the White and Black population, Asian population shows onset of type 2 diabetes at a lower BMI [11], thus suggesting that the cut-off values of BMI for predicting obesity-related adverse events vary geographically. Hence, extrapolating the results of previous studies conducted in non-Asians to Asians, including Japanese, becomes difficult [12].
Previous studies have reported inconsistent results on the association between BMI and the risk of disability in older adults, irrespective of the geographical region [13,14,15,16,17]. Higher BMI is associated with higher frailty levels [5, 18], while frailty also bears a strong association with disability [19]. Reportedly, overweight and obesity are inversely associated with mortality [20, 21] and falls [22] in individuals with frailty. Thus, some excess body weight is associated with longevity among older adults, especially in individuals with frailty. However, to the best of our knowledge, differences in the association of BMI with the age at overall, disability, and disability-free survival between older adults with and without frailty have not been thoroughly examined.
Our study objectives were (1) to evaluate the dose–response association between BMI and disability risk, and (2) to investigate the relationships between BMI and age at overall, disability, and disability-free survival in older adults with and without frailty. We hypothesised that both higher and lower BMI ranges would be associated with disability. Therefore, because BMI is associated with longevity [20, 21], less years with disability-free survival [14], we speculated that older adults with frailty with a higher BMI would have longer survival with disability than that of those with other BMI ranges.
Materials and methods
Study population and assessment of baseline characteristics
The Kyoto-Kameoka study is a population-based, prospective cohort study of individuals aged ≥65 years, residing in Kameoka City, Kyoto Prefecture, Japan. The details of this cohort study have been reported previously [6, 18, 21, 23,24,25,26]. Briefly, to conduct an appraisal of all residents of Kameoka City aged ≥65 years on 1 July 2011, a municipal employee in charge of the survey selected qualified candidates based on the individual’s name, sex, and date of birth; this information was obtained from the Basic Resident Register maintained by Kameoka City Hall (Fig. 1). The Needs in the Sphere of Daily Life survey (baseline survey), which includes the self-reported height, body weight, and frailty assessment tools [Kihon Checklist (KCL)], was mailed to the residents of Kameoka on 29 July 2011. Of these potential candidates (n = 18,231), 13,294 responded to the survey (response rate: 72.9%) by mail. We excluded individuals with incomplete responses to the KCL (n = 1,722), missing BMI data (n = 603), those who reported implausible BMI data [BMI < 14 or ≥40 kg/m2; n = 42) [18, 21], whose data could not obtained owing to relocation from the city (n = 16), and those who had disability at the commencement of follow-up (n = 679). Finally, 10,232 participants (5459 women; 4773 men) were included in the study.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the research ethics committees of the National Institutes of Biomedical Innovation, Health, and Nutrition (NIBIOHN-76-2), Kyoto University of Advanced Science (20-1), and Kyoto Prefectural University of Medicine (RBMR-E-363). Informed consent were received by mail from each participant along with the completed questionnaires.
Evaluation of BMI
BMI was calculated by dividing the self-reported body weight by the height squared (kg/m2). Previously, we found no significant difference between the BMI calculated from self-reported height and body weight and the objectively measured values in a sub-cohort of the Kyoto-Kameoka Study through clustered random sampling (mean difference: 0.5 kg/m2 in women and 0.4 kg/m2 in men; Pearson’s rank correlation coefficient between the BMI values: 0.912 for women, 0.916 for men) [18]. The interclass correlation coefficients, employed as a reproducibility scale of the self-reported BMI obtained from the baseline and additional surveys, were 0.888 and 0.910 for women and men, respectively [18]. For the Bland-Altman plot of agreement between self-reported and measured BMI, there was no variation in agreement in both sexes based on the BMI magnitude (Supplementary Fig. 1). We classified participants into the following BMI categories according to a previous study [21]: <18.5, 18.5–21.4, 21.5–24.9, 25.0–27.4, and ≥27.5 kg/m2. This is because if using the international BMI cut-off value for obesity (≥BMI 30 kg/m2) in our older Japanese population [27], only few would be considered as individuals with obesity (181 out of 10,232 [1.8%]). Furthermore, people with a ≥BMI 25 kg/m2 are defined as having obesity in Japan [27].
Assessment of frailty status
Frailty was defined as a score ≥7 on the 25-item, validated self-administered KCL [24, 28]. The KCL, based on a deficit accumulation model [6], assesses frailty from a multidimensional perspective, including cognitive, social, and depressive factors, in addition to physical aspects. The KCL encompasses seven subdomains: instrumental activities of daily living disability, malnutrition, physical inactivity, oral dysfunction, cognitive ___domain, socialisation ___domain, and depression. Difficulty in each activity or function is assigned one point, with the KCL score varying from 0 (no frailty) to 25 (high frailty). We have previously confirmed the good predictive ability of the KCL for frailty, defined by the revised Japanese version of the Cardiovascular Health Study criteria according to the Fried phenotype model in a sub cohort of the Kyoto-Kameoka study, that measured grip strength and gait speed (area under the receiver operating characteristic curve = 0.861; sensitivity = 76.2%; specificity = 79.9%) [24]. A prospective study demonstrated a dose response-dependent, strong log-linear relationship between the KCL score and mortality [6].
Disability and mortality status
Disability incidence was identified using the long-term care insurance system’s nationally unified database in Japan [29]. This system defines disability ( ≥ support level 1) as a condition in which some assistance is required for performing instrumental daily living activities. Local government officials performed in-person assessment of daily functioning in candidate individuals with disability using a 74-item questionnaire, based on the activities of daily living. Based on the questionnaire results and the physician’s opinion, the candidates’ functional disability level was determined by the Long-Term Care Insurance Certification Committee, comprising of academic experts in healthcare and welfare. This disability level information was provided by Kameoka City Hall officials. The survival status was assessed using data from the Basic Resident Register maintained by the Kameoka City Hall, collected between 30 July 2011 and 30 November 2016. Residents whose records were removed for administrative purposes or those who had moved out of the municipality were censored.
Other covariates
All covariates were obtained from questionnaire data from a baseline survey [26]. We collected data on the following basic characteristics: smoking status (“Do you smoke?”: almost daily; sometimes; used to, but quit; never); drinking status (“Do you drink alcohol?”: almost daily, sometimes, almost never, never); sleep duration (minutes); living status (“What is your family structure?”: living alone, living with family, other); education attainment (years); socioeconomic status (“Financially, how does your life feel currently?”: hard, somewhat hard, somewhat easy, easy); oral status (“Do you use dentures?”: yes, no); taking medication (number); and chronic disease [“Do you have a disease (presence of hypertension, stroke, heart disease, diabetes, hyperlipidaemia, gastrointestinal disease, respiratory disease, urological diseases, and cancer]?”: yes, no). Comorbidity scores were calculated from the data obtained on the nine comorbidity statuses. The summed value yielded a total score ranging from 0 (no comorbidity) to 9 (poor status). The previous week’s physical activity (PA) and sitting time per day were evaluated using the International Physical Activity Questionnaire-Short Form.
Statistical analysis
The participants’ descriptive statistics for continuous and categorical variables were presented as the mean with standard deviation and frequency with proportion, respectively. Missing indicators were created if information on covariates pertaining to smoking status (n = 195; 1.9%), alcohol consumption (n = 161; 1.6%), PA (n = 228; 2.2%), sitting time (n = 1075; 10.5%), sleep time (n = 484; 4.7%), family structure (n = 709; 6.9%), educational attainment (n = 1034; 10.1%), socioeconomic status (n = 369; 3.6%), denture use (n = 111; 1.1%), and medications (n = 558; 5.5%) was missing.
We calculated each participant’s person-years of follow-up from the date on which BMI was obtained to the date of disability and death, relocation from the study area, or end of follow-up, whichever occurred first. The rate of disability and all-cause mortality for each BMI group was presented as the number of events per 1000 person-years. We employed a multivariate Cox proportional hazards model that included baseline covariates to adjust for confounders associated with BMI and mortality. The assumptions of the Cox proportional hazards model were confirmed using the Schoenfeld residual test (p = 0.255). The results of these analyses were presented as hazard ratios (HRs) with 95% confidence intervals (CIs), calculated using the 21.5–24.9 kg/m2 BMI group as reference, based on previous studies [18, 21, 30]. The p-value of the linear trend was calculated by considering BMI exposure as a continuous variable. Furthermore, to evaluate the curvilinear relationship between BMI and disability, we used a restricted cubic spline model with three knots (10th, 50th, and 90th percentiles) based on the distribution of baseline BMI. These results were presented as HRs (95% CI), with the HR calculated using BMI = 23.0 kg/m2 as reference [21]. The statistical significance of nonlinearity was assessed using Wald’s test to compare the likelihood ratio of the spline model with the linear model, and p < 0.05 indicated a statistically significant non-linear relationship between the exposure and outcome [21, 25].
Furthermore, 50th percentile differences (PDs) in age at disability and death were calculated according to the BMI groups using the Laplace regression model. The PDs represented differences in the period until the first 50% of events occurred. This analysis adjusted covariates for model 2 using the ‘laplacereg’ command in STATA [31]. Median PDs (months) for overall and disability-free survival in each BMI group were calculated using the 21.5–24.9 kg/m2 group as reference. To calculate the duration of survival with disability, we calculated the difference of the 50th PDs of disability and death events using the following equation: 50th PD of overall survival—50th PD of disability-free survival. If the results were greater than 0 (value is +), we inferred that the duration of survival with disability was longer; if lower than 0, participants were more likely to die before disability incidence.
We performed sensitivity analysis using the following three methods: (1) to eliminate the possibility of reverse causal relationships, we excluded disability events (406 men and 712 women) recorded in the first 2 years of follow-up, (2) we performed a similar analysis using a dataset wherein missing values for covariates were replaced with multiple imputations, and (3) we used the multivariable sub-distribution hazard model approach proposed by Fine and Gray as the competing risk model. Multiple imputation analysis comprised the results of pooled analyses of 20 datasets, created with random numbers using the multiple-imputation method to replace the missing values of covariates using the ‘mi estimate’ command in STATA. All missing values were presumed to be missing at random. As mortality events might compete with these relationships when assessing the relationship between the exposure variables and the incidence of disability, disability was set as the event of interest and mortality as a competing event in competing risk model.
Multivariate analysis, which averts multicollinearity, was conducted by modelling potential confounders reported previously [20,21,22]. Model 1 was adjusted for age (continuous), sex (women or men), and population density ( ≥ 1000 or <1000 people/km2). Model 2 was adjusted for all variables from model 1 plus smoking status (never-smoker, past-smoker, current-smoker, missing), alcohol consumption (never-drinker, almost never-drinker, current-drinker, missing), PA ( < 150, 150–299, ≥300 min/week, missing), sitting time ( < 5, 5 to <7, 7 to <9, ≥9 h/d, missing), sleep duration (<360, 360 to <420, 420 to <480, ≥480 min/d, missing), family structure (living alone, living with others, missing), education level ( ≤ 9, 10–12, ≥13 years, missing), economic status (high, low, missing), denture use (yes, no, missing), medication use (none, 1, 2, 3, 4, ≥5, missing), number of chronic diseases (continuous), and frailty status (yes or no).
Statistical analyses were performed using STATA MP, Version 15.0 (StataCorp LP, College Station, TX, USA), and a two-tailed probability of <5% was considered significant.
Results
Table 1 presents the participants’ characteristics according to the BMI categories. Participants with BMI ≥ 27.5 kg/m2 were younger, and the proportion of current smokers, denture users, medication non-users, and high economic status was lower than in individuals with BMI < 18.5 kg/m2. The prevalence of frailty in the BMI < 18.5, 18.5–21.4, 21.5–24.9, 25.0–27.4, and ≥27.5 kg/m2 groups was 61.6%, 40.6%, 34.7%, 36.9% and 51.0%, respectively; thus, the prevalence of frailty was high in individuals with high and low BMI. These results were similar to those of the prevalence of subdomains, such as physical, cognitive, and depression, included in the KCL (Supplementary Table 1). Participants excluded from the analysis were older and were predominantly women compared to those who were included (Supplementary Table 2).
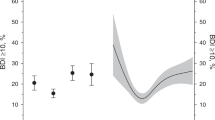
The relationships between BMI and disability are depicted in Figs. 2A, 3A. The median follow-up period was 5.3 years (interquartile range: 4.2–5.3 years). The total follow-up period was 45,472 person-years; 2348 (22.9%) incident disabilities occurred during the study period. After adjusting for confounders, such as medical history and lifestyle, individuals in the lower and higher BMI groups had a higher HR for disability than did those in the BMI = 21.5–24.9 kg/m2 group. Similar results were achieved using the restricted cubic spline model (Fig. 3A). The spline analysis model fit the data better than did the linear regression analysis (Akaike information criterion: 39,268 vs 39,287). A strong dose–response-dependent, log linear relationship was observed between KCL scores and disability risk (Fig. 3B), and individuals with frailty had a higher HR for disability than did those without (HR: 2.42, 95% CI: 2.18–2.68).
A total participants (B) frailty stratified model. Model 1 was adjusted for age, sex, and population density; Model 2 was additionally adjusted for smoking status, alcohol consumption, physical activity, sitting time, sleep duration, family structure, educational level, economic status, denture use, medication use, chronic disease count and/or frailty status. The X-axis of the plot is on a log scale. BMI body mass index, CI confidence interval, HR hazard ratio, PY person-years, Ref reference.
Body mass index and disability risk among (A) total participants, individuals with (C) frailty and (D) non-frailty. B frailty level and disability risk in total participants. The histogram shows the distribution of body mass index or Kihon checklist score. Solid lines represent hazard ratios, and dashed lines represent 95% confidence intervals (CIs). The hazard ratio was calculated based on a body mass index of 23.0 kg/m2 and Kihon checklist score of 0 point as reference. The adjustment factors are age, sex, population density, smoking status, alcohol consumption status, physical activity, sitting time, sleep time, family structure, educational attainment, economic status, denture use, medication use, number of chronic diseases, and/or frailty status.
Figures 2B and 3C, D depict the relationship between BMI and disability in individuals with and without frailty. Higher and lower BMI were associated with the risk for disability in such individuals. Similar results were obtained in the sensitivity analyses (Supplementary Tables 3–5). In the spline model, older adults with and without frailty exhibited the lowest HR for disability within a BMI range of 22.5–23.5 kg/m2 (Fig. 3C, D).
The relationships between BMI and 50th PDs in age at disability and death are shown in Fig. 4A, B and Supplementary Table 6. For the overall study population, the 50th PDs (95% CIs) in age at overall survival were 0 month (reference) for BMI = 21.5–24.9 kg/m2, −17.3 (−22.1 – −12.4) months for BMI < 18.5 kg/m2, and −7.6 (–11.4 to –3.8) months for BMI = 18.5–21.4 kg/m2 (Fig. 4A). Compared to BMI = 21.5–24.9 kg/m2, age at disability free-survival was –7.1 (–11.0 to –3.2) months shorter for BMI < 18.5 kg/m2 and –9.0 (–14.5 to −3.4) months shorter for BMI ≥ 27.5 kg/m2. Participants with BMI < 18.5 kg/m2 were more likely to die before disability incidence (survival with disability: –10.2 months) and those with BMI ≥ 27.5 kg/m2 had longer survival with disability (12.5 months). In the frailty status-stratified analyses, even a frail individual with optimal BMI faced a shorter of disability-free survival than did non-frail individuals with higher and lower BMI, respectively (Fig. 4B). In addition, individuals with frailty (27.2 months) with higher BMI had longer survival with disability than did those without (6.2 months).
A total participants (B) frailty stratified model. Results are presented as percentile differences (PDs) (95% confidence interval [CI]). To calculate the duration of survival with disability, we calculated the difference in the 50th PDs of disability and death events using the following equation: 50th PD of overall survival—50th PD of disability-free survival. If the results are greater than 0 (value is +), the duration of survival with disability is inferred to be longer. If the value is lower than 0, the participant is more likely to die before disability incidence. The adjustment factors are age, sex, population density, smoking status, alcohol consumption status, physical activity, sitting time, sleep time, family structure, educational attainment, economic status, denture use, medication use, number of chronic diseases, and/or frailty status.
Discussion
This study demonstrated that higher and lower BMI was associated with disability: older adults with BMI ≥ 27.5 kg/m2 had longer survival with disability, but those with BMI < 18.5 kg/m2 were more likely to die before disability incidence. Moreover, individuals with frailty and higher BMI had longer survival with disability than did those without frailty. To the best of our knowledge, this is the first study to demonstrate the existence of a dose–response relationship between BMI and disability in older individuals and that the duration of overall, disability, and disability-free survival differs with respect to BMI and frailty status.
We found that the HR for disability was the lowest at a BMI of 22.5–23.5 kg/m2 in older adults with and without frailty. Although results are inconsistent on the relationship between BMI and disability [13,14,15,16,17], a meta-analysis showed that BMI within the range of 23.0–28.0 kg/m2 was associated with the lowest HR for death and disability [32]. Given that Asians have a lower BMI optimal for disease prevention than do non-Asians [11], this finding approximates our results. Additionally, our findings indicated that participants with BMI < 18.5 kg/m2 were more likely to die before disability incidence and those with BMI ≥ 27.5 kg/m2 had longer survival with disability, especially those with frailty. Our previous [21] and other Japanese cohort studies [30] showed that BMI in the range of 23.0–24.0 kg/m2 and 22.0–24.9 kg/m2 is associated with the lowest mortality risk in older adults. Similar results were reported by two meta-analyses [33, 34]. Our previous [21] and other studies [20] showed that a higher BMI is inversely associated with mortality in individuals with frailty but not in those without, thus supporting our results and suggesting that higher BMI is associated with longer survival with disability among older adults, especially in individuals with frailty. Therefore, based on the current and previous studies [21, 30, 32, 33, 35], the optimal BMI range associated with a healthy life expectancy is 22.5–24.0 kg/m2, irrespective of the frailty status in older adults.
Although the mechanisms underlying the U-shaped relationships between BMI and disability are unclear, based on the results of previous studies, two factors associated with energy balance may be held accountable. BMI reflects the dynamic equilibrium of energy balance between energy intake and total energy expenditure [36]. First, a lower BMI may reflect lower energy intake because energy intake lower than the total energy expenditure leads to weight loss. Energy intake must be commensurate with energy requirements to maintain nutritional status and skeletal muscle mass in older adults [37]. Individuals with lower BMI cannot fully store skeletal muscle mass and fat [38], resulting in inadequate energy reserves available for mobilisation in acute stress events. Skeletal muscle mass is inversely associated with disability risk [39], possibly contributing to disability risk because of a lack of energy.
Second, a higher BMI may reflect lower energy expenditure or higher energy intake. PA accounts for approximately 20–35% of the total energy expenditure [40], and people with obesity and overweight are more likely to engage in a lower amount of PA. Objectively measured PA bears an inverse association with disability in older adults [41, 42]. An intervention study showed that the disability prevention effect of PA is more pronounced in older adults with poor lower limb function [43]. High energy intake [23] and obesity [18] are associated with frailty. The Wisconsin Primate Calorie Restriction study indicated that calorie restriction prevented a decline in PA and reduced the incidence of frailty compared to that with ad libitum feeding in rhesus monkeys [44], suggesting that high energy intake and obesity may accelerate ageing, thus supporting our results.
Our study strength was that it evaluated the relationship between BMI and disability in older individuals aged ≥65 years from Kameoka city’s large-scale population-based cohort, using validated self-reported BMI [18] and frailty assessment tools [24]. This approach probably minimised misclassification of BMI groups and frailty status due to self-report bias. However, the study contained certain methodological limitations. First, baseline survey data could not be collected from all residents who were sent the questionnaire. We observed differences in individual characteristics, such as age and sex, between residents who were excluded due to missing exposure or outcome variables and those who were included in the study. This suggests that our participants were more health-conscious than were older people in the general population. Second, BMI evaluation was performed only at baseline, and the participants’ BMI might have changed during the follow-up period, leading to misclassification of BMI and potentially attenuating the relationship between BMI and disability in the exposure assessment. Despite this limitation, this study affirmed the relationship between BMI and disability in both the main and sensitivity analyses. Third, the follow-up period was relatively short; this may result in overestimation of the relationships between the exposure variables and outcomes and inversion of the causal relationship [45] because the HR estimated from the analysis may change with time. However, we confirmed proportional hazards for the relationship between BMI and disability, and the sensitivity analysis that excluded disability events occurring after first 2 years of follow-up showed similar results. Finally, although our study adjusted for several confounders, residual confounders may persist in the association between steps and disability.
Although some epidemiological studies have shown a U-shaped relationship between BMI and all-cause mortality in adults [30, 33, 35, 46], the optimal BMI associated with the lowest risk of mortality increased with age in both Japanese [30, 46] and other populations [33]. Reportedly, a higher BMI may confer protection against the risk of mortality in older individuals with frailty than in those without [20, 21], thus supporting the obesity paradox in older adults with frailty [47]. However, it has been also argued that the obesity paradox is induced by the following two biases [48]: 1) the influence of differences in individuals characteristics, such as people with obesity being younger and more well-nourished than are people without obesity, and 2) the influence of collider stratification bias, which leads to a spurious association between exposure factors and outcomes by stratifying by a third variable, that is associated with both the exposure variable (BMI) and the confounding factor and is downstream from both. Additionally, individuals who are constantly overweight or are undergoing constant weight loss have a higher mortality risk compared to that of those with a constant normal weight, thereby suggesting that the obesity paradox may not be observed when weight trajectories are considered [49]. Therefore, when evaluating the relationship between BMI and mortality risk, the obesity paradox should be re-evaluated using trajectories and changes in body weight and frailty levels as exposure or mediate variables, because reportedly the onset of frailty during the observation period may be a mediating mechanism between weight loss in midlife and increased mortality risk in old age [49].
We found that even individuals with frailty with optimal BMI showed a significantly shorter disability-free survival than did older individuals without frailty with a BMI of 21.5–24.9 kg/m2, suggesting that optimal BMI does not completely offset the risk of frailty-related adverse events. This suggested that the obesity paradox may not exist if it were to consider disability rather than mortality, as individuals with frailty and obesity have the longest duration of survival with disability. Maintaining the optimal BMI and improving frailty may contribute not only to prolonging life expectancy, but also shortening survival with disability in older adults. Frailty is a reversible condition wherein the individual can return to a healthy state through appropriate lifestyle intervention programmes [50]; therefore, mitigating frailty levels should be prioritised over attaining the optimal BMI in older individuals with frailty. Moreover, early detection of frailty using frailty screening tools by healthcare manager or professionals is necessary in clinical and public health settings. As the prevalence of subdomains related to frailty varies according to BMI [18], interventions should be personalized based on an individual’s BMI and frailty aspects.
Conclusion
The BMI range with the lowest HR for disability was 22.5–23.5 kg/m2 in older adults with and without frailty, and higher and lower BMI was associated with disability. Older adults with BMI ≥ 27.5 kg/m2 had longer survival with disability, but those with BMI < 18.5 kg/m2 were more likely to die before disability incidence. This study suggests that reversal of frailty should be prioritised because even individuals with frailty with an optimal BMI face a higher risk of disability than do individuals without frailty with higher or lower BMI.
Data availability
The datasets described in the manuscript will be made available by the corresponding author ([email protected]), TY ([email protected]), and YY ([email protected]) on reasonable request.
References
Blodgett JM, Rockwood K, Theou O. Changes in the severity and lethality of age-related health deficit accumulation in the USA between 1999 and 2018: a population-based cohort study. Lancet Healthy Longev. 2021;2:e96–e104. https://doi.org/10.1016/S2666-7568(20)30059-3
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752–62. https://doi.org/10.1016/S0140-6736(12)62167-9
Fried LP, Cohen AA, Xue QL, Walston J, Bandeen-Roche K, Varadhan R. The physical frailty syndrome as a transition from homeostatic symphony to cacophony. Nat Aging. 2021;1:36–46. https://doi.org/10.1038/s43587-020-00017-z
Fan J, Yu C, Guo Y, Bian Z, Sun Z, Yang L, et al. Frailty index and all-cause and cause-specific mortality in Chinese adults: a prospective cohort study. Lancet Public Health. 2020;5:e650–e60. https://doi.org/10.1016/S2468-2667(20)30113-4
Hanlon P, Nicholl BI, Jani BD, Lee D, McQueenie R, Mair FS. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: a prospective analysis of 493 737 UK Biobank participants. Lancet Public Health. 2018;3:e323–e32. https://doi.org/10.1016/S2468-2667(18)30091-4
Watanabe D, Yoshida T, Yamada Y, Watanabe Y, Yamada M, Fujita H, et al. Combined use of two frailty tools in predicting mortality in older adults. Sci Rep. 2022;12:15042 https://doi.org/10.1038/s41598-022-19148-x
Dupre ME, Gu D, Warner DF, Yi Z. Frailty and type of death among older adults in China: prospective cohort study. BMJ. 2009;338:b1175 https://doi.org/10.1136/bmj.b1175
O’Caoimh R, Sezgin D, O’Donovan MR, Molloy DW, Clegg A, Rockwood K, et al. Prevalence of frailty in 62 countries across the world: a systematic review and meta-analysis of population-level studies. Age Ageing. 2021;50:96–104. https://doi.org/10.1093/ageing/afaa219
Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. N Engl J Med. 1983;309:1218–24. https://doi.org/10.1056/NEJM198311173092005
Collaboration N C D R F. Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–4. https://doi.org/10.1038/s41586-019-1171-x
Caleyachetty R, Barber TM, Mohammed NI, Cappuccio FP, Hardy R, Mathur R, et al. Ethnicity-specific BMI cutoffs for obesity based on type 2 diabetes risk in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2021;9:419–26. https://doi.org/10.1016/S2213-8587(21)00088-7
Rao G, Powell-Wiley TM, Ancheta I, Hairston K, Kirley K, Lear SA, et al. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: A scientific statement from the American Heart Association. Circulation. 2015;132:457–72. https://doi.org/10.1161/CIR.0000000000000223
Lv YB, Yuan JQ, Mao C, Gao X, Yin ZX, Kraus VB, et al. Association of body mass index with disability in activities of daily living among Chinese adults 80 years of age or older. JAMA Netw Open. 2018;1:e181915 https://doi.org/10.1001/jamanetworkopen.2018.1915
Zhang S, Tomata Y, Tanji F, Sugawara Y, Tsuji I. The relationship between body mass index and disability-free survival in elderly Japanese: the Ohsaki Cohort 2006 Study. Int J Obes (Lond). 2019;43:2254–63. https://doi.org/10.1038/s41366-019-0359-3
Diehr P, O’Meara ES, Fitzpatrick A, Newman AB, Kuller L, Burke G. Weight, mortality, years of healthy life, and active life expectancy in older adults. J Am Geriatr Soc. 2008;56:76–83. https://doi.org/10.1111/j.1532-5415.2007.01500.x
Walter S, Kunst A, Mackenbach J, Hofman A, Tiemeier H. Mortality and disability: the effect of overweight and obesity. Int J Obes (Lond). 2009;33:1410–8. https://doi.org/10.1038/ijo.2009.176
Al Snih S, Ottenbacher KJ, Markides KS, Kuo YF, Eschbach K, Goodwin JS. The effect of obesity on disability vs mortality in older Americans. Arch Intern Med. 2007;167:774–80. https://doi.org/10.1001/archinte.167.8.774
Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Kimura M, Kyoto-Kameoka StudyG. A U-shaped relationship between the prevalence of frailty and body mass index in community-dwelling Japanese older adults: The Kyoto-Kameoka study. J Clin Med. 2020;9:1367 https://doi.org/10.3390/jcm9051367
Satake S, Shimokata H, Senda K, Kondo I, Toba K. Validity of total Kihon checklist score for predicting the incidence of 3-year dependency and mortality in a community-dwelling older population. J Am Med Dir Assoc. 2017;18:552e1–6. https://doi.org/10.1016/j.jamda.2017.03.013
Jayanama K, Theou O, Godin J, Mayo A, Cahill L, Rockwood K. Relationship of body mass index with frailty and all-cause mortality among middle-aged and older adults. BMC Med. 2022;20:404 https://doi.org/10.1186/s12916-022-02596-7
Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Miyachi M, Kimura M. Frailty modifies the association of body mass index with mortality among older adults: Kyoto-Kameoka study. Clin Nutr. 2024;43:494–502. https://doi.org/10.1016/j.clnu.2024.01.002
Boutin E, Natella PA, Schott AM, Bastuji-Garin S, David JP, Paillaud E, et al. Interrelations between body mass index, frailty, and clinical adverse events in older community-dwelling women: The EPIDOS cohort study. Clin Nutr. 2018;37:1638–44. https://doi.org/10.1016/j.clnu.2017.07.023
Watanabe D, Yoshida T, Nanri H, Watanabe Y, Date H, Itoi A, et al. Association between the prevalence of frailty and doubly labeled water-calibrated energy intake among community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2021;76:876–84. https://doi.org/10.1093/gerona/glaa133
Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Miyachi M, Kimura M. Validation of the Kihon Checklist and the frailty screening index for frailty defined by the phenotype model in older Japanese adults. BMC Geriatr. 2022;22:478 https://doi.org/10.1186/s12877-022-03177-2
Watanabe D, Yoshida T, Watanabe Y, Yamada Y, Miyachi M, Kimura M. Dose-response relationships between objectively measured daily steps and mortality among frail and nonfrail older adults. Med Sci Sports Exerc. 2023;55:1044–53. https://doi.org/10.1249/MSS.0000000000003133
Yamada Y, Nanri H, Watanabe Y, Yoshida T, Yokoyama K, Itoi A, et al. Prevalence of frailty assessed by Fried and Kihon checklist indexes in a prospective cohort study: Design and demographics of the Kyoto-Kameoka longitudinal study. J Am Med Dir Assoc. 2017;18:733 e7–15. https://doi.org/10.1016/j.jamda.2017.02.022
Ogawa W, Hirota Y, Miyazaki S, Nakamura T, Ogawa Y, Shimomura I, et al. Definition, criteria, and core concepts of guidelines for the management of obesity disease in Japan. Endocr J. 2024;71:223–31. https://doi.org/10.1507/endocrj.EJ23-0593
Ambagtsheer RC, Visvanathan R, Dent E, Yu S, Schultz TJ, Beilby J. Commonly used screening instruments to identify frailty among community-dwelling older people in a general practice (primary care) setting: A study of diagnostic test accuracy. J Gerontol A Biol Sci Med Sci. 2020;75:1134–42. https://doi.org/10.1093/gerona/glz260
Yamada M, Arai H. Long-term care system in Japan. Ann Geriatr Med Res. 2020;24:174–80. https://doi.org/10.4235/agmr.20.0037
Hozawa A, Hirata T, Yatsuya H, Murakami Y, Kuriyama S, Tsuji I, et al. Association between body mass index and all-cause death in Japanese population: Pooled individual participant data analysis of 13 cohort studies. J Epidemiol. 2019;29:457–63. https://doi.org/10.2188/jea.JE20180124
Bottai M, Orsini N. A command for Laplace regression. Stata J. 2013;13:302–14. https://doi.org/10.1177/1536867x1301300204
Jiang M, Zou Y, Xin Q, Cai Y, Wang Y, Qin X, et al. Dose-response relationship between body mass index and risks of all-cause mortality and disability among the elderly: A systematic review and meta-analysis. Clin Nutr. 2019;38:1511–23. https://doi.org/10.1016/j.clnu.2018.07.021
Aune D, Sen A, Prasad M, Norat T, Janszky I, Tonstad S, et al. BMI and all cause mortality: systematic review and non-linear dose-response meta-analysis of 230 cohort studies with 3.74 million deaths among 30.3 million participants. BMJ. 2016;353:i2156 https://doi.org/10.1136/bmj.i2156
Berrigan D, Troiano RP, Graubard BI. BMI and mortality: the limits of epidemiological evidence. Lancet. 2016;388:734–6. https://doi.org/10.1016/S0140-6736(16)30949-7
Global B M I M C, Di Angelantonio E, Bhupathiraju ShN, Wormser D, Gao P, Kaptoge S, et al. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–86. https://doi.org/10.1016/S0140-6736(16)30175-1
Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–37. https://doi.org/10.1016/S0140-6736(11)60812-X
Volkert D, Beck AM, Cederholm T, Cruz-Jentoft A, Goisser S, Hooper L, et al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr. 2019;38:10–47. https://doi.org/10.1016/j.clnu.2018.05.024
Liu C, Wong PY, Chung YL, Chow SK, Cheung WH, Law SW, et al. Deciphering the “obesity paradox” in the elderly: A systematic review and meta-analysis of sarcopenic obesity. Obes Rev. 2023;24:e13534 https://doi.org/10.1111/obr.13534
Wang DXM, Yao J, Zirek Y, Reijnierse EM, Maier AB. Muscle mass, strength, and physical performance predicting activities of daily living: a meta-analysis. J Cachexia Sarcopenia Muscle. 2020;11:3–25. https://doi.org/10.1002/jcsm.12502
Butte NF, Ekelund U, Westerterp KR. Assessing physical activity using wearable monitors: measures of physical activity. Med Sci Sports Exerc. 2012;44:S5–12. https://doi.org/10.1249/MSS.0b013e3182399c0e
Glass NL, Bellettiere J, Jain P, LaMonte MJ, LaCroix AZ, Women’s HealthI. Evaluation of light physical activity measured by accelerometry and mobility disability during a 6-year follow-up in older women. JAMA Netw Open. 2021;4:e210005 https://doi.org/10.1001/jamanetworkopen.2021.0005
Dunlop DD, Song J, Semanik PA, Sharma L, Bathon JM, Eaton CB, et al. Relation of physical activity time to incident disability in community dwelling adults with or at risk of knee arthritis: prospective cohort study. BMJ. 2014;348:g2472 https://doi.org/10.1136/bmj.g2472
Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA. 2014;311:2387–96. https://doi.org/10.1001/jama.2014.5616
Yamada Y, Kemnitz JW, Weindruch R, Anderson RM, Schoeller DA, Colman RJ. Caloric restriction and healthy life span: Frail phenotype of nonhuman primates in the Wisconsin National Primate Research Center Caloric Restriction Study. J Gerontol A Biol Sci Med Sci. 2018;73:273–8. https://doi.org/10.1093/gerona/glx059
Hernan MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. https://doi.org/10.1097/EDE.0b013e3181c1ea43
Matsuo T, Sairenchi T, Iso H, Irie F, Tanaka K, Fukasawa N, et al. Age- and gender-specific BMI in terms of the lowest mortality in Japanese general population. Obesity (Silver Spring). 2008;16:2348–55. https://doi.org/10.1038/oby.2008.342
Lv Y, Mao C, Gao X, Ji JS, Kraus VB, Yin Z, et al. The obesity paradox is mostly driven by decreased noncardiovascular disease mortality in the oldest old in China: a 20-year prospective cohort study. Nat Aging. 2022;2:389–96. https://doi.org/10.1038/s43587-022-00201-3
Banack HR, Stokes A. The ‘obesity paradox’ may not be a paradox at all. Int J Obes (Lond). 2017;41:1162–3. https://doi.org/10.1038/ijo.2017.99
Strandberg TE, Stenholm S, Strandberg AY, Salomaa VV, Pitkala KH, Tilvis RS. The “obesity paradox,” frailty, disability, and mortality in older men: a prospective, longitudinal cohort study. Am J Epidemiol. 2013;178:1452–60. https://doi.org/10.1093/aje/kwt157
Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347:1068–74. https://doi.org/10.1056/NEJMoa020423
Acknowledgements
We wish to thank all the members of the Kyoto-Kameoka Study Group for their valuable contributions. We are grateful to the administrative staff of Kameoka City and Kyoto Prefecture. We wish to express our gratitude to all participants for their cooperation in this study. We also wish to thank Editage (www.editage.jp) for English language editing.
Funding
The Kyoto-Kameoka Study was conducted with JSPS KAKENHI and supported by research grants provided to Misaka Kimura (grant number: 24240091), Yosuke Yamada (grant number: 15H05363), Daiki Watanabe (grant number: 21K17699), and Tsukasa Yoshida (grant numbers: 22H03525 and 23K24782); grant and administrative support by the Kyoto Prefecture Community-based Integrated Elderly Care Systems Promotion Organization since 2011; and Kameoka City under the programme of the Long-term Care Insurance and Planning Division of the Health and Welfare Bureau for the Elderly, the Ministry of Health, Labour and Welfare, and the WHO Collaborating Centre on Community Safety Promotion.
Author information
Authors and Affiliations
Contributions
DW, YY, and MK designed the study; TY, YW, YY, and MK conducted the research; DW analysed the data; DW wrote the first draft of the paper; and DW had primary responsibility for the final content. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, D., Yoshida, T., Watanabe, Y. et al. Is a higher body mass index associated with longer duration of survival with disability in frail than in non-frail older adults?. Int J Obes 49, 348–356 (2025). https://doi.org/10.1038/s41366-024-01681-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-024-01681-6
This article is cited by
-
One-year outcomes in sepsis: a prospective multicenter cohort study in Japan
Journal of Intensive Care (2025)