Abstract
Objectives
To evaluate the association between an exclusive human milk diet (EHMD) and motor function impairment at three years of corrected age among infants born before 32 weeks of gestation.
Methods
We conducted a retrospective study between 2018 and 2021. We assigned to the EHMD group infants who received an EHMD for ≥75% of the days between the first day of diet fortification and 33 6/7 weeks postmenstrual age. We used inverse propensity scores to balance potential confounders and developed a mixed-effects logistic regression model to assess the association.
Results
After adjusting for demographics and morbidities, an EHMD was associated with a reduced risk of motor function impairment, with an odds ratio of 0.74 (95% CI: 0.56–0.98, p = 0.036).
Conclusions
An EHMD is associated with a decrease in the odds of early childhood motor function impairment among infants born before 32 weeks of gestation.
Similar content being viewed by others
Introduction
Human milk-based nutrition is the standard of care for infants born before 32 weeks and very low birth weight infants [1]. To meet the unique nutritional needs and growth of these infants, multi-nutrient fortification of either mother’s own milk (MOM) or donor human milk (DHM) is recommended [2, 3]. Fortification of MOM or DHM can be achieved using either bovine milk-based fortifier (BMBF) or human milk-based fortifier (HMBF). An exclusive human milk diet (EHMD) consists of MOM or DHM as the primary milk source, which is supplemented with HMBF. EHMD was found to be associated with a decrease in the incidence of necrotizing enterocolitis (NEC), especially in those settings where the NEC rates are high, as well as other morbidities such as late-onset sepsis, days on mechanical ventilation, retinopathy of prematurity (ROP), and bronchopulmonary dysplasia (BPD) [4,5,6,7,8], although these benefits have not been confirmed in randomized controlled trials (RCTs) [9, 10]. There is conflicting evidence on the relationship between EHMD and short-term postnatal growth, with numerous studies showing reduced growth in association with EHMD use [7, 9, 11,12,13,14]. Using a retrospective cohort of infants born before 32 weeks at Kaiser Permanente Southern California (KPSC), we also showed reduced length growth in infants offered an EHMD, after accounting for confounders and adjusting for neonatal morbidities. In a post-hoc analysis, we observed comparable length growth and improved weight growth in infants offered an EHMD fortified directly to a caloric density of 26 kcal/oz [15].
Neurodevelopmental outcomes in early childhood are often used as indicators to evaluate the quality of care given to preterm infants in the NICUs. The late second and early third trimesters are crucial for rapid brain development, but this process is significantly interrupted by preterm birth before 32 weeks of gestation. Various factors, such as prolonged respiratory support, severity of illness, acute brain injury, nutritional intake, and growth during this critical period all influence neurodevelopmental outcomes. Slower growth has been correlated with higher risks of neurodevelopmental impairment [16, 17]. In contrast, the use of human milk and breastfeeding have been shown to improve neurodevelopmental outcomes in both term and preterm infants [18,19,20]. Specific components of human milk, such as long-chain polyunsaturated fatty acids (LC-PUFA), lactoferrin, and human milk oligosaccharides (HMOs), are believed to play a role in supporting neurodevelopment [18, 21,22,23].
The impact of an EHMD on early childhood neurodevelopmental outcomes has been reported in the literature but with conflicting results [12, 24,25,26]. In a pragmatic trial by Hopperton et al., infants weighing less than 1250 g between 2014 and 2016 were block-randomized to receive either HMBF or BMBF. Neurodevelopmental outcomes assessed at 18 months of corrected age revealed no significant differences between the two groups [24]. Conversely, a multicenter retrospective study conducted across six NICUs between 2006 and 2010 reported superior cognitive outcomes at 18–22 months of age, as assessed by the Bayley Scales of Infant and Toddler Development (BSID), among infants fed an EHMD. However, no significant differences were observed in language or motor outcomes. These findings remained consistent in both univariate analyses and after adjusting for factors such as birth weight, sex, enteral feeding, and NEC [26]. Colacci et al. found no differences in BSID cognitive scores between groups [12]. The study by Bergner et al. reported neurodevelopmental outcomes but did not include a non-EHMD group for comparison [25]. In this study, we leverage an established KPSC cohort [15] to examine the association between EHMD and motor function impairment at three years of age.
Methods
Study design, eligibility criteria, and oversight
We conducted a multicenter retrospective study involving infants born at less than 32 weeks of gestation between January 1, 2018, and August 31, 2021, across 13 NICUs within KPSC. This cohort represents a subset of our previously published study [15], including only those infants who had reached three years of corrected age by the time when the analysis started. Infants with major congenital anomalies or those who were no longer Kaiser Permanente members by three years of corrected age, either due to mortality or member attrition, were excluded. Additionally, we excluded infants without documented feeding information by 34 weeks postmenstrual age (PMA) or with missing data on caloric density. The study was approved by the KPSC Institutional Review Board (IRB), which waived the requirement for informed consent (IRB #: 13350). All methods were performed in accordance with the relevant guidelines and regulations.
Data collection
Demographic and morbidity data were collected from the Clarity database, a reporting database of Epic electronic healthcare records, or the research database at KPSC. No artificial intelligence or natural language processing algorithms were used for data extraction. Morbidity and outcome diagnoses were based on international classification of diseases-tenth edition (ICD-10) codes. Maternal variables collected include age, smoking status during pregnancy, delivery modes, antenatal corticosteroid administration, hypertensive disorders of pregnancy (HDP, including preeclampsia, eclampsia, HELLP syndrome), fetal growth restriction (FGR), gestational diabetes mellitus (GDM), obesity, and placental abruption. Neonatal variables extracted include birth hospital, sex, race/ethnicity, gestational age (GA), length of stay, APGAR scores at 1 and 5 minutes, IVH, ROP, NEC, spontaneous intestinal perforation (SIP), BPD (based on the 2019 Jensen criteria [27]), dexamethasone administration, ibuprofen/indomethacin administration, and periventricular leukomalacia. To identify cases of NEC and SIP, we first screened Neonatology encounter records for the following ICD-10 codes: P77.2 (Stage 2 NEC), P77.3 (Stage 3 NEC), P77.9 (NEC unspecified), and P78.0 (perinatal intestinal perforation), followed by manual chart reviews to confirm the diagnosis. Notably, for cases of NEC managed medically, only those with clear documentation of pneumatosis intestinalis on abdominal x-rays and treatment with antibiotics were classified as having NEC. SIP rather than surgical NEC was diagnosed if the infant was not fed enterally or only received trophic feeds when free air was observed on the abdominal x-rays and surgical intervention was performed. Infants with a diagnostic code of P77.1 (Stage 1 NEC) were not classified as having NEC.
Anthropometric data collected include weight, length, and head circumference measurements from birth to 33 6/7 weeks PMA. Weight was typically measured daily, whereas length and head circumference were typically measured weekly, but the frequency may change depending on clinical needs. The method for conducting the measurements was unavailable. The birth measurements were the measurements closest to the infants’ birth dates. Birth measurement percentiles as well as weight and length growth trajectory percentiles were calculated based on the 2023 Postnatal Growth Charts for Preterm Infants [28].
NICUs that implemented EHMD used the Prolacta products as part of their routine care. Since NICUs transitioned infants from HMBF to BMBF at varying PMAs after 34 0/7 weeks, we limited our analysis to diet data collected before 34 0/7 weeks PMA to assign infants to either the EHMD or non-EHMD group. Daily diet order was used to determine whether the infant received EHMD for the day or not. The percentage of EHMD was calculated as follows:
Following the same grouping criteria as our previous study, infants who received EHMD for ≥75% of the days were assigned to the EHMD group [15]. The remainder of the infants were assigned to the non-EHMD group regardless of whether the non-EHMD feeds were preterm formula or MOM/DHM fortified with BMBF. Caloric density data were collected from nursing documentation of each feed in the flowsheet.
Infants were classified as having motor function impairment if they had any of the following ICD-10 codes in their medical charts―M62.9 (hypotonia), G80.X (cerebral palsy), or F82 (specific developmental disorder of motor function)―after NICU discharge and before reaching 3 years of corrected age, and the diagnoses remained active at three years of corrected age.
Statistical analysis
Continuous variables were presented as median [interquartile range (IQR)] or mean ± sd. Categorical variables were presented as number (percentage). The rank sum test or the t test was used to compare continuous variables, and the Chi-squared test was used for categorical variables. Standardized mean differences (SMD) were presented in demographic summarization of the confounders. P-values were presented for all comparisons. A P value < 0.05 was considered statistically significant.
We used inverse propensity weighting (IPW) to account for imbalance in perinatal confounders between the EHMD vs. non-EHMD groups as a result of variations in EHMD implementation protocols across NICUs. IPW is a statistical method used to mimic a randomized experiment in observational data. It works by assigning weights to each patient based on the inverse of their propensity score—the probability of receiving a particular treatment given their characteristics. This reweighting balances the groups so they’re more comparable. In other words, it gives more weight to individuals who are underrepresented and less to those overrepresented, reducing bias in estimating treatment effects. Propensity scores were derived using generalized boosted regression modeling to estimate the population average treatment effect [29]. Variables screened for inclusion in propensity score estimation were potential confounders before the beginning of diet fortification in typical modern neonatal practices, including maternal obesity, HDP, GDM, FGR, chorioamnionitis, antenatal corticosteroids, infant race/ethnicity, birth GA, infant sex, birth measurement (weight, length, and head circumference) percentiles, as well as 1- and 5-min APGAR scores. Those variables with a p value < 0.1 between the EHMD and non-EHMD group in the unweighted cohort were included in IPW.
A weighted mixed-effects logistic regression model was developed to assess the risk of motor function impairment in association with EHMD. Additional fixed-effect variables include those demographic and neonatal morbidity variables that showed a difference between the EHMD and non-EHMD groups. Medical centers were included as a random-effect term to account for outcome variations among centers. The risk of motor function impairment was presented as odds ratio (OR) and its 95% confidence interval (CI).
Results
Infant characteristics
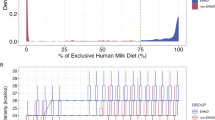
We identified 1058 infants who met the selection criteria, with 800 infants in the non-EHMD group and 258 in the EHMD group. Infants in the EHMD group were born earlier (median GA of 27 weeks for the EHMD group vs. 30 weeks for the non-EHMD group). Their perinatal characteristics are listed in Table 1. Birth weight and length percentiles were significantly lower among infants in the EHMD group. Fewer infants in the EHMD group were born vaginally and large for gestational age (LGA), and more infants were born small for gestational age (SGA). Both 1-min and 5-min APGAR scores were lower in the EHMD group. Infants in the EHMD group had 100% (IQR: 92–100%) of their total fortification days on EHMD, while infants in the non-EHMD group had 0% (IQR: 0–0%) of their total fortification days on EHMD (Fig. 1A). Maximum caloric intake was significantly higher in the EHMD group (26 [IQR: 26–28] kcal/oz) compared to that in the non-EHMD group (24 [24-26] kcal/oz), with p < 0.001 (Fig. 1B).
A A histogram illustrating the distribution of the percentage of days infants received an EHMD between the first day of fortification and 33 6/7 weeks postmenstrual age. The EHMD group was arbitrarily defined as having ≥ 75% of days on an EHMD, whereas the non-EHMD group was defined as having <75% of days on an EHMD. B Boxplots comparing caloric density by day for the first 21 days after diet fortification began between the EHMD and non-EHMD groups. Each boxplot displays the median (thick horizontal line), 25th and 75th percentiles (box edges), and whiskers extending to 1.5 times the interquartile range above and below the percentiles. Dashed lines (dark grey for EHMD, light grey for non-EHMD) indicate the median caloric density for each group over time.
Maternal obesity, chorioamnionitis, antenatal corticosteroids, mode of delivery, GA, birth weight, length, and head circumference percentiles, and 1- and 5-min APGAR scores were used for IPW. The weighted population has 1820 infants, with 1015 in the non-EHMD group and 805 in the EHMD group. The perinatal characteristics of the weighted population are listed in Table 2. The SMD for GA (from 0.759 to 0.115), birth weight percentile (from 0.331 to 0.106), and birth length percentile (from 0.177 to 0.055) became much smaller after IPW. However, the differences between the two groups remained statistically significant (p < 0.05). Antenatal corticosteroid use was also significantly different between the two groups, although 94.3% of the weighted population in the non-EHMD group and 97.9% in the EHMD group received antenatal corticosteroids. The other variables were balanced between the two groups.
Postnatal morbidities are listed in Table 3. Infants in the EHMD group were more likely to have IVH (31.2% in the EHMD group vs. 19.6% in the non-EHMD group, p < 0.001), although the differences in grade 3/4 IVH were non-significant between the two groups (6.9% in the EHMD vs. 4.9% in the non-EHMD group). Infants in the EHMD group were also more likely to have grade 2/3 BPD (14.6% in EHMD vs. 9.9% in non-EHMD, p = 0.039). Length of stay was longer among infants in the EHMD group (60 [IQR: 44–87] days) than in the non-EHMD group (51 [IQR:36–75] days), with p = 0.001.
Assessing the risks of motor function impairment
Confounders that remained imbalanced after IPW and morbidities that were significantly different between the two groups in the weighted population were identified and included in the model for adjusting. These factors consisted of GA (categorized into 22–25, 26–27, 28–29, and 30–31 groups to improve model fit), birth weight percentile, grade 1/2 and grade 3/4 IVH, ibuprofen/indomethacin use, grade 2/3 BPD, antenatal and postnatal corticosteroid use (Tables 2, 3). The adjusted OR for motor function impairment was 0.74 (95% CI: 0.56–0.98, p = 0.036) in the EHMD group compared to the non-EHMD group (Fig. 2). Additionally, lower GA, grade 3/4 IVH, and grade 2/3 bronchopulmonary dysplasia were also independently associated with a significantly higher risk of motor function impairment.
A forest plot illustrating odds ratios (represented by black dots) and 95% confidence intervals (depicted as whiskers) for motor function impairment, comparing the EHMD and non-EHMD groups, along with significant confounders and covariates. The vertical line at an odds ratio of 1 indicates no effect. Confounders and covariates adjusted in the model include gestational age groups, antenatal corticosteroids, grade 1/2 intraventricular hemorrhage, grade 3/4 intraventricular hemorrhage, ibuprofen/indomethacin treatment, postnatal corticosteroids, grade 2/3 bronchopulmonary dysplasia, and birth weight percentile.
Discussion
In this study, we assessed the association between EHMD and motor function impairment, defined based on having a documented diagnosis of hypotonia, cerebral palsy, or specific developmental disorder of motor function that remained active at three years of corrected age. We applied IPW to balance potential perinatal confounders. We then used a multivariable mixed-effects model to (1) adjust for residual confounding as well as significant differences in morbidity covariates, and (2) account for outcome variations across medical centers. Our analysis showed that EHMD was independently associated with a reduced risk of motor function impairment.
Human milk influences neurodevelopment through multiple mechanisms mediated by the gut-brain axis [23]. Among its components, lactoferrin, one of the most abundant whey proteins, has been linked to brain size in preterm infants [30]. Its anti-inflammatory properties may help mitigate adverse neurodevelopmental outcomes associated with systemic inflammation [31, 32]. Another critical group of components in human milk is LC-PUFA, including docosahexaenoic acid (DHA) and arachidonic acid (ARA), which are key structural components of central nervous system cell membranes and play a significant role in regulating their function [33]. ARA and DHA accumulate rapidly during the third trimester and the first postnatal months in term infants. De novo synthesis of ARA exceeds that of DHA in preterm infants, making DHA more of an essential fatty acid in these vulnerable infants [34]. High-dose DHA supplementation in infants born before 29 weeks of gestation has been associated with improved full-scale intelligence quotient at 5 years of corrected age [35]. HMOs are another group of molecules implicated in human milk’s contribution to neurodevelopment [23, 36]. With over 200 identified structures, HMO concentrations vary depending on geographic ___location, maternal genetics, and the lactation period. Indigestible by infants, HMOs are instead utilized by the neonatal gut microbiome to suppress pathogenic microorganism growth. They also exhibit anti-inflammatory properties by downregulating pro-inflammatory cytokine secretion by intestinal epithelial cells, and play a role in strengthening the intestinal epithelial barrier [37]. Functionally, human milk use is associated with increased white matter development, cortical thickness, as well as better BSID and intelligence quotient scores [18].
Despite the presence of various components in human milk that are associated with improved neurodevelopment, the benefits of an EHMD remain a subject of debate. An EHMD comprises MOM or DHM fortified with HMBF, with the fortifier derived from banked DHM. Compared to mature MOM, banked DHM has reduced macro- and micronutrient content as well as lower caloric density. [38,39,40,41]. The concentrations of anti-inflammatory lactoferrins and immunoglobulins were also substantially reduced in banked DHM [40]. Moreover, the human milk microbiome is likely inactivated during the pasteurization process, leading to distinct intestinal microbiome signatures in preterm infants fed MOM-based diets compared to those fed DHM-based diets [42]. Nonetheless, a recent biochemical study demonstrated that DHM fortified with HMBF restored certain components, including lactoferrin, and achieved higher concentrations of protein and fat [43]. Demonstrating a correlation between an EHMD and reduced motor function impairment, our current study suggests that the bioactive components in an EHMD collectively offer significant benefits to preterm infants. Notably, our study compared EHMD vs. non-EHMD, which is different from the recently published MILK trial where no differences in neurodevelopmental outcomes were observed between infants randomized to DHM fortified with BMBF vs. preterm formula [44]. Infants from the MILK trial would both be included in the non-EHMD group.
In this study, we used physician-entered diagnostic codes in the electronic health record to identify motor function impairment. Although the list of diagnostic codes is not exhaustive, and this approach differs from conventional methods such as BSID or other standardized tests to evaluate motor developmental milestones, it may offer a more pragmatic alternative. Physician diagnoses are likely informed by a combination of assessment modalities, including standardized testing, parental or caregiver input, and physical examinations. Notably, the prevalence of motor function impairment in our cohort aligns with published rates based on standardized testing [45]. We focused on motor function impairment because, based on developmental cascades, motor development tends to present earlier than language and cognitive development, and contributes to language development [46, 47]. Furthermore, early motor impairment appears to correlate with later deficits in high-level functioning, likely due to damage to shared neural pathways caused by prematurity [48]. Motor impairment is also less influenced by social determinant of health [49].
Several factors likely explain the low NEC rate in our cohort. The study only included infants who survived to 3 years of corrected age, excluding those infants with NEC who had died before then. Additionally, we used ICD codes to identify NEC and SIP cases, a method that may be affected by coding errors. Furthermore, most infants were born to mothers receiving regular antenatal care within the Kaiser Permanente system, which includes antenatal corticosteroids and prompt treatment for chorioamnionitis, interventions known to reduce NEC risk.
This study had several limitations. First, its retrospective design inherently limited the availability of detailed enteral and parenteral nutrition data for adjustment. This issue was compounded by the absence of a standardized protocol for introducing an EHMD across NICUs. Although confounder balancing was conducted to weight-adjust each infant, residual confounding persisted between the non-EHMD and EHMD groups in the weighted population. Additionally, while using diagnostic codes to define motor function impairment has its advantages, as previously noted, the diagnosis itself may be subjective and influenced by the biases of the diagnosing physician. Finally, the accuracy of diagnostic code entries could not be verified.
Conclusion
Infants born before 32 weeks of gestation fed an EHMD demonstrate reduced risk of motor function impairment, highlighting its benefits beyond NICU hospitalization for this vulnerable population. A prospective study is warranted to further validate these findings. Additionally, identifying the bioactive components in EHMD that contribute to its neurodevelopmental advantages could provide valuable insights into the unique composition of the banked DHM concentrate used in EHMD.
Data availability
Deidentified individual participant data may be requested with a formal utilization plan, pending approval by the Institutional Review Board of Southern California Kaiser Permanente.
References
Parker MG, Stellwagen LM, Noble L, Kim JH, Poindexter BB, Puopolo KM, et al. Promoting human milk and breastfeeding for the very low birth weight infant. Pediatrics. 2021;148:e2021054272.
Brown JVE, Lin L, Embleton ND, Harding JE, McGuire W. Multi-nutrient fortification of human milk for preterm infants. Cochrane Libr. 2020;2020. https://doi.org/10.1002/14651858.cd000343.pub4
Embleton ND, Jennifer Moltu S, Lapillonne A, van den Akker CHP, Carnielli V, Fusch C, et al. Enteral nutrition in preterm infants (2022): a position paper from the ESPGHAN committee on nutrition and invited experts. J Pediatr Gastroenterol Nutr. 2023;76:248–68.
Sullivan S, Schanler RJ, Kim JH, Patel AL, Trawöger R, Kiechl-Kohlendorfer U, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr. 2010;156:562–7.e1.
Hair AB, Hawthorne KM, Chetta KE, Abrams SA. Human milk feeding supports adequate growth in infants ≤ 1250 grams birth weight. BMC Res Notes. 2013;6:459.
Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl-Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr. 2013;163:1592–95.e1.
Hair AB, Peluso AM, Hawthorne KM, Perez J, Smith DP, Khan JY, et al. Beyond necrotizing enterocolitis prevention: improving outcomes with an exclusive human milk-based diet. Breastfeed Med. 2016;11:70–4.
Lucas A, Boscardin J, Abrams SA. Preterm infants fed cow’s milk-derived fortifier had adverse outcomes despite a base diet of only mother’s own milk. Breastfeed Med. 2020;15:297–303.
O’Connor DL, Kiss A, Tomlinson C, Bando N, Bayliss A, Campbell DM, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born weighing <1250 g: a randomized clinical trial. Am J Clin Nutr. 2018;108:108–16.
Jensen GB, Domellöf M, Ahlsson F, Elfvin A, Navér L, Abrahamsson T. Effect of human milk-based fortification in extremely preterm infants fed exclusively with breast milk: a randomised controlled trial. EClinicalMedicine. 2024;68:102375.
Huston RK, Markell AM, McCulley EA, Pathak M, Rogers SP, Sweeney SL, et al. Decreasing necrotizing enterocolitis and gastrointestinal bleeding in the neonatal intensive care unit: The role of donor human milk and exclusive human milk diets in infants ≤1500 g birth weight. Infant Child Adolesc Nutr. 2014;6:86–93.
Colacci M, Murthy K, DeRegnier R-AO, Khan JY, Robinson DT. Growth and development in extremely low birth weight infants after the introduction of exclusive human milk feedings. Am J Perinatol. 2017;34:130–7.
Eibensteiner F, Auer-Hackenberg L, Jilma B, Thanhaeuser M, Wald M, Haiden N. Growth, feeding tolerance and metabolism in extreme preterm infants under an exclusive human milk diet. Nutrients. 2019;11:1443.
Velumula PK, Elbakoush F, Elfadeel H, Lulic-Botica M, Natarajan G, Bajaj M. Comparative growth outcomes in preterm infants fed either mother’s own milk or donor human milk. Breastfeed Med. 2023;18:300–6.
Chou F-S, Zhang J, Nguyen C, Faison GM, Thompson LR, Villosis MFB, et al. The impact of exclusive human milk diet on short-term growth of very preterm infants. J Perinatol. 2024;44. https://doi.org/10.1038/s41372-024-01980-w
Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61.
Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128:e899–906.
Lechner BE, Vohr BR. Neurodevelopmental outcomes of preterm infants fed human milk: a systematic review. Clin Perinatol. 2017;44:69–83.
Belfort MB, Knight E, Chandarana S, Ikem E, Gould JF, Collins CT, et al. Associations of maternal milk feeding with Neurodevelopmental Outcomes at 7 years of age in former preterm infants. JAMA Netw Open. 2022;5:e2221608.
Zhang R, Ying E, Wu X, Qin H, Guo Y, Guo X, et al. A systematic review and meta-analysis of breastfeeding and neurodevelopmental outcomes in preterm infant. Front Public Health. 2024;12:1401250.
Silveira RC, Corso AL, Procianoy RS. The influence of early nutrition on neurodevelopmental outcomes in preterm infants. Nutrients. 2023;15:4644.
Berger PK, Ong ML, Bode L, Belfort MB. Human milk oligosaccharides and infant neurodevelopment: a narrative review. Nutrients. 2023;15. https://doi.org/10.3390/nu15030719
Perez KM, Strobel KM, Hendrixson DT, Brandon O, Hair AB, Workneh R, et al. Nutrition and the gut-brain axis in neonatal brain injury and development. Semin Perinatol. 2024;48:151927.
Hopperton KE, O’Connor DL, Bando N, Conway AM, Ng DVY, Kiss A, et al. Nutrient enrichment of human milk with human and bovine milk-based fortifiers for infants born <1250 g: 18-month neurodevelopment follow-up of a randomized clinical trial. Curr Dev Nutr. 2019;3:nzz129.
Bergner EM, Shypailo R, Visuthranukul C, Hagan J, O’Donnell AR, Hawthorne KM, et al. Growth, body composition, and neurodevelopmental outcomes at 2 years among preterm infants fed an exclusive human milk diet in the neonatal intensive care unit: A pilot study. Breastfeed Med. 2020;15:304–11.
Hair AB, Patel AL, Kiechl-Kohlendorfer U, Kim JH, Schanler RJ, Hawthorne KM, et al. Neurodevelopmental outcomes of extremely preterm infants fed an exclusive human milk-based diet versus a mixed human milk + bovine milk-based diet: a multi-center study. J Perinatol. 2022;42:1485–8.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9.
Chou F-S, Yeh H-W, Clark RH. A comparative study of postnatal anthropometric growth in very preterm infants and intrauterine growth. Nat Commun. 2023;14:5626.
twang: Toolkit for Weighting and Analysis of Nonequivalent Groups. In: Comprehensive R Archive Network (CRAN) [Internet]. [cited 10 Oct 2024]. Available: https://cran.r-project.org/web/packages/twang/index.html
Atayde AMP, Kapoor NR, Cherkerzian S, Olson I, Andrews C, Lee ACC, et al. Lactoferrin intake from maternal milk during the neonatal hospitalization and early brain development among preterm infants. Pediatr Res. 2024;96:159–64.
O’Shea TM, Allred EN, Kuban KCK, Dammann O, Paneth N, Fichorova R, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J Pediatr. 2012;160:395–401.e4.
Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75:376–80.
Georgieff MK, Innis SM. Controversial nutrients that potentially affect preterm neurodevelopment: essential fatty acids and iron. Pediatr Res. 2005;57:99R–103R.
Lapillonne A, Moltu SJ. Long-chain polyunsaturated fatty acids and clinical outcomes of preterm infants. Ann Nutr Metab. 2016;69:35–44.
Gould JF, Makrides M, Gibson RA, Sullivan TR, McPhee AJ, Anderson PJ, et al. Neonatal docosahexaenoic acid in preterm infants and intelligence at 5 years. N Engl J Med. 2022;387:1579–88.
Soyyılmaz B, Mikš MH, Röhrig CH, Matwiejuk M, Meszaros-Matwiejuk A, Vigsnæs LK. The mean of milk: A review of human milk oligosaccharide concentrations throughout lactation. Nutrients. 2021;13:2737.
Rousseaux A, Brosseau C, Le Gall S, Piloquet H, Barbarot S, Bodinier M. Human milk oligosaccharides: Their effects on the host and their potential as therapeutic agents. Front Immunol. 2021;12:680911.
Wojcik KY, Rechtman DJ, Lee ML, Montoya A, Medo ET. Macronutrient analysis of a nationwide sample of donor breast milk. J Am Diet Assoc. 2009;109:137–40.
COMMITTEE ON NUTRITION, SECTION ON BREASTFEEDING, COMMITTEE ON FETUS AND NEWBORN. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics. 2017;139:e20163440.
Hård A-L, Nilsson AK, Lund A-M, Hansen-Pupp I, Smith LEH, Hellström A. Review shows that donor milk does not promote the growth and development of preterm infants as well as maternal milk. Acta Paediatr. 2019;108:998–1007.
Gates A, Hair AB, Salas AA, Thompson AB, Stansfield BK. Nutrient composition of donor human milk and comparisons to preterm human milk. J Nutr. 2023;153:2622–30.
Parra-Llorca A, Gormaz M, Alcántara C, Cernada M, Nuñez-Ramiro A, Vento M, et al. Preterm gut microbiome depending on feeding type: Significance of donor human milk. Front Microbiol. 2018;9. https://doi.org/10.3389/fmicb.2018.01376
Philip RK, Romeih E, Bailie E, Daly M, McGourty KD, Grabrucker AM, et al. Exclusive human milk diet for extremely premature infants: A novel fortification strategy that enhances the bioactive properties of fresh, frozen, and pasteurized milk specimens. Breastfeed Med. 2023;18:279–90.
Colaizy TT, Poindexter BB, McDonald SA, Bell EF, Carlo WA, Carlson SJ, et al. Neurodevelopmental outcomes of extremely preterm infants fed donor milk or preterm infant formula: a randomized clinical trial. JAMA. 2024;331:582–91.
Evensen KAI, Ustad T, Tikanmäki M, Haaramo P, Kajantie E. Long-term motor outcomes of very preterm and/or very low birth weight individuals without cerebral palsy: A review of the current evidence. Semin Fetal Neonatal Med. 2020;25:101116.
Valentini NC, de Borba LS, Panceri C, Smith BA, Procianoy RS, Silveira RC. Early detection of cognitive, language, and motor delays for low-income preterm infants: A Brazilian cohort longitudinal study on infant neurodevelopment and maternal practice. Front Psychol. 2021;12:753551.
Kobaş M, Kızıldere E, Doğan I, Aktan-Erciyes A, Demir-Lira ÖE, Akman İ, et al. Motor skills, language development, and visual processing in preterm and full-term infants. Curr Psychol. 2023;42:12463–75.
Van Hus JW, Potharst ES, Jeukens-Visser M, Kok JH, Van Wassenaer-Leemhuis AG. Motor impairment in very preterm-born children: links with other developmental deficits at 5 years of age. Dev Med Child Neurol. 2014;56:587–94.
Brumbaugh JE, Vohr BR, Bell EF, Bann CM, Travers CP, McGowan EC, et al. Early-life outcomes in relation to social determinants of health for children born extremely preterm. J Pediatr. 2023;259:113443.
Funding
F-SC is supported by the Clinician Investigator Program (2023-2024) from the Division of Clinician Research of the Department of Research and Evaluation at Kaiser Permanente Southern California.
Author information
Authors and Affiliations
Contributions
F-SC conceptualized and designed the study, supervised data collection, performed the initial analysis, interpreted data, and drafted the initial and the revised manuscript. JZ collected data, assisted in data analysis, and critically reviewed the initial and revised drafts of the manuscript. MFBV contributed to data interpretation and critically reviewed the initial and revised drafts of the manuscript for important intellectual content. AL participated in study design, supervised data interpretation, and critically reviewed the initial and revised drafts of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This data-only study was approved by the Kaiser Permanente Southern California Institutional Review Board, with an exemption from the requirement for informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chou, FS., Zhang, J., Villosis, M.F.B. et al. Exclusive human milk diet is associated with lower risk of motor function impairment at three years of corrected age. J Perinatol (2025). https://doi.org/10.1038/s41372-025-02296-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-025-02296-z