Abstract
Non-invasive brain–computer interfaces (NI-BCIs) have garnered significant attention due to their safety and wide range of applications. However, developing non-invasive electroencephalogram (EEG) electrodes that are highly sensitive, comfortable to wear, and reusable has been challenging due to the limitations of conventional electrodes. Here, we introduce a simple method for fabricating semi-dry hydrogel EEG electrodes with antibacterial properties, enabling long-term, repeatable acquisition of EEG. By utilizing N-acryloyl glycinamide and hydroxypropyltrimethyl ammonium chloride chitosan, we have prepared electrodes that not only possess good mechanical properties (compression modulus 65 kPa) and anti-fatigue properties but also exhibit superior antibacterial properties. These electrodes effectively inhibit the growth of both Gram-negative (E. coli) and Gram-positive (S. epidermidis) bacteria. Furthermore, the hydrogel maintains stable water retention properties, resulting in an average contact impedance of <400 Ω measured over 12 h, and an ionic conductivity of 0.39 mS cm−1. Cytotoxicity and skin irritation tests have confirmed the high biocompatibility of the hydrogel electrodes. In an N170 event-related potential (ERP) test on human volunteers, we successfully captured the expected ERP signal waveform and a high signal-to-noise ratio (20.02 dB), comparable to that of conventional wet electrodes. Moreover, contact impedance on the scalps remained below 100 kΩ for 12 h, while wet electrodes became unable to detect signals after 7–8 h due to dehydration. In summary, our hydrogel electrodes are capable of detecting ERPs over extended periods in an easy-to-use manner with antibacterial properties. This reduces the risk of bacterial infection associated with prolonged reuse and expands the potential of NI-BCIs in daily life.

Similar content being viewed by others
Introduction
Brain–computer interface (BCI) is a system that directly connects a human or animal brain to an external device, designed to enable real-time information exchange between the brain and the device1,2. BCI technology has evolved into a promising application across various domains including healthcare, human augmentation, military combat, and edutainment3,4,5. BCI systems can be classified as invasive, semi-invasive, and non-invasive based on the way the EEG signals are acquired and where the electrodes are implanted in the brain6,7. Among them, non-invasive brain–computer interfaces are characterized by safety, non-invasiveness, and convenience, making them highly promising for daily life applications and receiving widespread attention8. The technical challenge of non-invasive EEG interfaces lies in acquiring high-precision EEG signals. Therefore, the selection of appropriate electrodes is vital, as it directly influences the quality and accuracy of the signals obtained9.
At the present time, non-invasive BCIs for acquiring EEG signals are typically categorized into three main types: wet electrodes, dry electrodes, and semi-dry electrodes10. Wet electrodes are considered the “gold standard” for EEG signal acquisition11. However, wet electrodes are prone to water loss, cannot be monitored for long periods of time, are complicated to handle, and need to be cleaned after use12. The use of conductive gel or viscous paste can also lead to hair contamination and potentially severe allergic reactions if there are scalp abrasions13. These inconveniences and discomforts limit the use of wet electrodes in daily life. Dry electrodes are straightforward to utilize, can be readily removed, and are suitable for repeated use14. However, dry electrodes are susceptible to hair interference during EEG signal acquisition, generating motion artefacts, and the quality of the acquired signals is often not accurate enough15. In addition, wearing dry electrodes for a long period of time can cause pain to the subject, which limits the total duration of wear16.
In response to the limitations of both wet and dry electrodes, semi-dry electrodes are increasingly being employed to enhance both wearing comfort and signal quality. Semi-dry electrodes integrate features from conventional wet and dry electrodes, addressing the need to reduce impedance without leaving behind conductive paste residue after use17. Flexible semi-dry electrodes require high electrical conductivity and compatibility with biological tissues. The physicochemical properties of hydrogels are analogous to those of biological tissues, rendering them an optimal composition for next-generation electrodes, which are becoming increasingly prevalent in biosensor applications18. For instance, Zhu et al. developed a novel stretchable piezoresistive strain sensor using a semi-interpenetrating network hydrogel formed by CMC micro sheets and PAAm through monomer polymerization. This sensor is capable of monitoring subtle vibrations and human motion19. Han et al. constructed a multichannel wearable electrode based on PVA-PVP-PDA NPs with excellent self-adhesion and electrical conductivity. This electrode can reliably and stably acquire prefrontal EEG signals and be used as a portable sustained attention assessment device in both scientific research and daily life20. Nevertheless, the self-adhesive hydrogel is incapable of capturing signals in regions, such as the hair-dense hindbrain functional area. K. Jakab and colleagues refined the production of polyvinyl alcohol–glycerol–NaCl hydrogel electrodes with high ionic conductivity. These electrodes offer favorable and satisfactory skin contact in hairy areas of the scalp, and their high glycerol content ensures reusability and a long shelf-life. However, the complexity and time-consuming nature of the electrode fabrication process, along with the large size of the finished product, present usability challenges21. Xue et al. designed and prepared a substrate-adhesive bilayer hydrogel EEG electrode that is capable of continuously recording EEG signals for 12 h. The electrode features a highly conductive layer with high electrical conductivity, low skin contact impedance, and high robustness. The electrode is straightforward to handle and can be recycled, which is anticipated to facilitate the utilization of non-invasive EEG electrodes in a multitude of everyday contexts22. Nevertheless, the experiment did not offer further validation of the anti-bacterial profile of the hydrogel electrode. It is therefore necessary to consider bacterial transmission in order to assess the potential for recycling the hydrogel. Due to its high-water content, hydrogel is susceptible to bacterial growth in humid environments23. This may result in the deterioration of device performance, moreover, may pose a health risk to users. Consequently, the creation of hydrogel electrodes with anti-bacterial properties has become a significant area of current research. Wang et al. developed a spongy cellulose aerogel flexible electrode doped with polypyrrole-coated silver nanowires to enhance its conductivity and anti-bacterial properties, thereby facilitating long-term high-quality electrophysiological monitoring24. However, the experiment did not employ the same aerogel electrode for long-term signal monitoring, and the long-term biosafety experimental validation was also inadequate.
As technology advances, there is a growing demand for antimicrobial properties in flexible electrodes, particularly in the context of wearable devices that come into direct contact with the skin. The incorporation of antimicrobial properties can effectively inhibit bacterial growth and reproduction, while also mitigating potential damage to the user’s skin25,26. However, there is a paucity of research exploring the incorporation of antibacterial properties into semi-dry hydrogel EEG electrodes. This is largely due to the inherent challenges associated with achieving a balance between three key factors: ensuring optimal electrical conductivity, providing a comfortable wearing sensation, and ensuring long-term stability. Tables S1 and S2 compare semi-dry hydrogel electrodes for EEG signal acquisition.
In this study, we designed and developed a tough semi-dry hydrogel electrode with antibacterial properties, capable of continuously acquiring high-quality EEG signals for up to 12 h or more. The superior antibacterial property enables multiple reuses of the hydrogel electrode. We characterized the microstructure and mechanical properties of the hydrogels and measured their electrical conductivity, including electrode–skin contact impedance and ionic conductivity. We performed 1000 cyclic voltammetry (CV) cycle tests using an electrochemical workstation. Charge storage capacitance (CSC) and charge injection capacity (CIC) at the hydrogel interface were characterized. Additionally, anti-bacterial tests confirmed its superior antibacterial performance, while cytotoxicity and allergic reaction tests demonstrated good biocompatibility. Furthermore, we compared the performance of the hydrogel electrodes with that of dry and wet electrodes on human volunteers, recording and testing real-time contact impedance over 12 h and conducting continuous N170 EEG experiments for 21 days to evaluate the hydrogel electrodes’ ability to effectively capture EEG signals.
Materials and methods
Chemicals
Poly (N-acryloyl glycinamide) (PNAGA) was purchased from Shanghai Famasitanda Biotechnology Co., Ltd. Hydroxypropyltrimethyl ammonium chloride chitosan (HACC) was purchased from Macklin. Potassium chloride (KCl) was purchased from Xilong Scientific. Glycerol (Gl) was purchased from Damao Chemical Reagent Factory. Lipidure-51 was purchased from Guangzhou Baolong International Trade Co., Ltd. Ammonium persulfate (APS) was purchased from Aladdin. All the chemicals were used as received without further purification.
Synthesis method of hydrogels
The composition of the prepared hydrogels is below and may be increased or decreased proportionally. If not otherwise specified, the hydrogel is prepared as a cylinder 10 mm in diameter and 8 mm in height.
Synthesis of conductive hydrogels: 0.4 g NAGA, 0.055 g KCl, 0.88 mL glycerol were dissolved in 1 mL of deionized water and degassed by ultrasonication until completely dissolved, then 0.0028 g APS was added as an initiator, mixed homogeneously and then poured into molds, illuminated in a UVLED curing light source for 60 s (power 100%), and then placed at 65 °C for 1.5 h to obtain a conductive hydrogel. This conductive hydrogel was used as a control group.
Synthesis of conductive hydrogels with anti-bacterial properties: dissolve 0.01, 0.02, 0.03, 0.04, and 0.05 g of HACC in 1 mL of deionized water to obtain 1, 2, 3, 4, and 5 wt% HACC solution, respectively, and then dissolve 0.4 g of NAGA, 0.055 g of KCl, 0.88 mL of glycerol in the abovementioned 1 mL of HACC solution, and then degas by ultrasonic degassing until complete dissolution. 0.0028 g of APS was added as an initiator, mixed homogeneously and poured into molds, illuminated in a UVLED curing light source for 60 s (power 100%), and then put into an oven at 65 °C for 1.5 h to obtain a conductive hydrogel with anti-bacterial properties. The conductive hydrogel with anti-bacterial properties was used as an experimental group.
Scanning electron microscope (SEM)
The morphology and microstructure of the hydrogel cross-section were obtained by SEM (SU8822 0) at an accelerating voltage of 5 kV. The prepared hydrogel samples were pre-cooled in liquid nitrogen, lyophilized at −80 °C, and then placed in a freeze-dryer for 3 days. The lyophilized samples were cut with a scalpel blade in order to form the cross-section, which was subsequently adhered to the sample stage with a double-sided electrician’s tape, spray-coated using platinum, scanned, and imaged with SEM.
Assembly of non-invasive hydrogel electrodes
Solidworks software was used to design the supports and molds of the non-invasive electrodes, the designed supports and molds were cast by 3D printing, Ag/AgCl buttons were assembled, the hydrogel prepolymer solution was poured into the molds, and the non-invasive hydrogel electrodes were obtained by UV irradiation and heat curing.
Characterization of mechanical properties
The mechanical properties of the hydrogels were evaluated using an MST, 50 N transducer with compression test samples of cylinders at a test rate of 5 mm/min, and the compression modulus was determined from the slope of the compression curve at 0–30% strain. At least four samples were used for each mechanical test. Furthermore, in order to assess the fatigue resistance and self-recovery characteristics of hydrogels, compression cycle tests were conducted. The specified end-point deformation was set at 4 mm (50%), with the tensile machine automatically compressing and releasing the hydrogel samples in each cycle.
Characterization of moisturizing properties
In this experiment, the effect of two common moisturizers (glycerol and lipidure-51) on the water retention properties of hydrogels was tested. The cylindrical hydrogels were placed in a ventilated environment at room temperature and the hydrogels were weighed for 12 h consecutively and for a long period of time (6 weeks), and the water retention rate of the hydrogels was calculated using the following formula:
W0 denotes the initial weight of the hydrogel, and Wt denotes the weight of the hydrogel after dehydration at time t.
Skin–electrode contact impedance
The skin–electrode contact impedance was measured using the four-electrode method. Fresh pork skin was placed in a refrigerator at −20 °C for spare time and thawed before use. The skin was washed with water to remove excess fat and oil, cut into rectangles 20 cm long and 5 cm wide, and fixed on a base plate. Under the slide of the measuring instrument, an electrode was mounted at 4 cm intervals so that it was located directly above the pig skin, the slide was moved downwards to keep the hydrogel electrode tightly attached to the pig skin, and then the circuit was connected according to the circuit diagram. The Eb-electrode was connected to the circuit by turning the switch with a voltage of 2 V. The data were recorded after the readings had stabilized. The same procedure is then performed when the Ea-electrode is connected to the circuit. The formula for calculating the skin–electrode contact impedance is as follows:
Vbc and VR are the indications of the voltmeter when the circuit is connected to Eb, V′bc, and V′R are the indications of the voltmeter when the circuit is connected to Ea and the resistance of R is 1000 Ω.
In this experiment, a total of five hydrogels with different KCl concentrations (0.25, 0.50, 0.75, 1.00, and 1.25 mol/L) were measured. At least three samples were used for each test. In addition, we tested the change in contact impedance of the wet and hydrogel electrodes for 12 h continuously at room temperature.
Electrochemical impedance spectroscopy (EIS)
EIS was performed using the CHI660E workstation. The test samples were cylinders with a diameter of 10 mm and a height of 3 mm, and at least three samples were used for each test. The hydrogel was clamped with two copper plates and tested in the frequency range of 0.1–105 Hz at a constant voltage of 5 mV. Meanwhile, the conductivity of the hydrogel was measured and was calculated as follows:
L, R, and A denote the length, resistance, and cross-sectional area of the hydrogel, respectively.
Next, to evaluate the long-term stability of the hydrogel electrode, the cyclic voltammetry (CV) curve of the hydrogel electrode was further investigated. The charge storage capacity (CSC) and charge injection capacity (CIC) of the hydrogel electrode were also measured.
Characterization of anti-bacterial properties
The anti-bacterial properties of hydrogels were investigated using Escherichia coli (E. coli, Gram-negative bacteria, ATCC 8739) and Staphylococcus epidermidis (S. epidermidis, Gram-positive bacteria, ATCC 12228) as bacterial models. E. coli was cultured in LB liquid/solid medium at 37 °C. S. epidermidis was cultured in NA liquid/solid medium at 37 °C. The control and experimental hydrogels used for testing were cylinders.
Anti-bacterial properties in solid media: Evaluation of the anti-bacterial activity by agar well diffusion method. Then, 5 μL of the fresh bacterial solution was diluted to 5 mL, and 5 μL of the diluted bacterial solution was aspirated and spread uniformly on the agar plate. The inactivated control and experimental hydrogels were placed in the center of the agar plate and placed into a bacterial incubator, the plate was observed after 24 h, and the diameter of the inhibition rings was measured and recorded. Each experiment was repeated four times.
Anti-bacterial properties in liquid media: Anti-bacterial activity was assessed by measuring optical density (OD). Fresh bacterial solution (5 μL) was added to a sterilized shake flask containing 5 mL of liquid medium. At the same time, a blank group was prepared with LB/NA liquid medium containing the bacterial solution and a blank control group with a pure LB/NA liquid medium without the bacterial solution. The bacterial solution (200 μL) was collected from each group at each time interval. The absorbance was measured at 600 nm, and four parallel experiments were performed for each group. And take out 5 μL of bacterial solution and dilute it 1000-fold, then take 10 μL of the diluted bacterial solution and spread it evenly on the agar plate, and observe the colony growth after 24 h.
Detection of anti-bacterial properties of hydrogels on the surface: The control and experimental hydrogel electrodes, worn for more than 3 days, were disassembled from the EEG experiments tested by the subjects without any treatment. The used hydrogels were then sealed in a bag and stored at room temperature, and the changes in the two groups of hydrogels were observed at various time points.
Cytotoxicity assay
The effects of control hydrogels and hydrogels with different HACC contents on mouse embryonic fibroblasts (3T3) were assessed by CCK-8 measurement of cell viability. Cylindrical hydrogels were irradiated with UV for 1 h in an ultra-clean bench for complete sterilization, and then the hydrogels were immersed in 8 mL of DMEM complete medium (10% fetal bovine serum, 1% penicillin/streptomycin) for 3 days. Control and raw hydrogel suspensions with different HACC contents were prepared, and hydrogel suspensions at a concentration of 5 mg/mL were prepared by dilution of the raw suspensions. These suspensions were used as a conditioned medium for the detection of cumulative hydrogel release or degradation products.
3T3 cells cultured with DMEM complete medium were inoculated into 96-well plates (100 μL per well) at a density of 10,000 cells/per well and placed in an incubator at 37 °C, 5% CO2. After 24 h, the well plates were removed, the medium was aspirated out, and 100 μL of the prepared hydrogel suspension was added to each well. The plates were then incubated for 6 h. After this incubation, 10 μL of CCK-8 reagent was added to each well under dark conditions, and the absorbance at 450 nm was measured using an enzyme marker after 1 h of dark incubation. The cell viability was calculated using the following formula:
As: The absorbance of the experimental wells (containing cells, medium, CCK-8 solution, and drug solution);
Ac: The absorbance of the control wells (containing cells, medium, CCK-8 solution, and no drug);
Ab1: The absorbance of the blank wells (containing medium, drug, CCK-8 solution, excluding cells);
Ab2: The absorbance of the blank wells (containing medium, CCK-8 solution, excluding cells and drugs).
Skin irritation test
Five male and five female Sprague-Dawley rats (200 g) were used to evaluate the potential skin irritation caused by the hydrogels. The animals were acclimatized for 1 week before beginning the animal experiments.
First, the hair on the back of the rats was shaved with a shaver, ensuring that the exposed area was free of visible hair. Four cylindrical hydrogels (10 mm in diameter and 3 mm in height) were then applied externally to the shaved area. Four male and four female rats were observed for 1, 3, 5, and 7 days, respectively. After the designated period, the dressings were removed, and the exposed skin was carefully examined for redness, swelling, or other signs of irritation. At the same time, one male and one female rat were selected as the blank control group and were only shaved. At the end of the experimental point in time, the skin under the dressing was peeled off and fixed with paraformaldehyde for hematoxylin and eosin (H&E) staining.
Hydrogel electrodes EEG signal acquisition
In this experiment, a 32-channel EEG signal acquisition system positioned according to an international 10–20 system was used to collect EEG signals, with CPz and GND as online references. Dry electrodes, wet electrodes, and hydrogel electrodes were fixed to the EEG cap using buttons, respectively. The EEG signals of the subjects were continuously collected using an eegoTM mylab amplifier (ANT Neuro, Enschede, The Netherlands) with a sampling frequency of 1000 Hz. These signals were analyzed to compare the stability of the EEG signals acquired from the three electrodes. Additionally, the 12-h real-time contact impedance of the electrodes was recorded using the eegoTM software.
N170 EEG experiment
The participants sat comfortably in a sound-proofed acoustic dark chamber. First, they ignored the meaning of the words and responded to key presses based on the color of the target word: pressing the ‘1’ key for red words and the ‘2’ key for blue words. This familiarized them with the procedure of the test. After achieving 75% accuracy, the test began. Participants responded to the color of the target word appearing on the screen within 2 s, and the test will take 7–8 min. Three healthy male subjects and one healthy female subject were recruited for this experiment, and all participants were guaranteed to have normal or corrected vision and not be color blind. Subsequently, we experimentally verified whether the hydrogel electrodes could be recovered for reuse. The above volunteers were asked to wear the same hydrogel electrode for 2 h per day, with an interval of about 3 days for the N170 mood-evoking experiment, which lasted for a total of 21 days.
The signal-to-noise ratio was calculated in the following way27: first, the signal 200 ms before stimulus onset was chosen as the baseline window (noise), and 100–250 ms after stimulus onset was used as the signal window (we focused on the negative signal around 170 ms). The signal peaks were obtained by calculating the absolute value of the minimum of each channel within the signal window, while the noise variance was the variance of the baseline window data (where N is the number of trials of the signal)
Results and discussion
Preparation and characterization of hydrogels
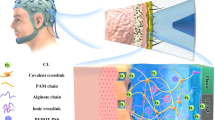
The objective of this study was to develop a new type of non-invasive hydrogel EEG electrode with strong toughness, superior anti-bacterial properties, excellent electrical conductivity, high biocompatibility, moisture retention, and low contact impedance. We first chose the polymerizable monomer N-acryloyl glycinamide (NAGA), which can be initiated directly and easily in water to form poly (N-acryloyl glycinamide) (PNAGA). The double amide group sequence in the side chain forms highly stable hydrogen-bonded domains between the two amide groups, causing strong physical cross-linking. As a result, a concentrated aqueous solution of PNAGA (≥10 wt%) can form a high-strength supramolecular polymer hydrogel28. Chitosan (CH) has considerable reactive amino and hydroxyl groups and can be chemically and physically cross-linked to form various types of CH-based hydrogels. CH can be chemically modified to prepare common derivatives, such as hydroxypropyltrimethyl ammonium chloride chitosan (HACC), carboxymethyl chitosan (CMCH), and hydroxy butyl chitosan (HBC)29. The quaternization of CH not only enhances its positive electronegativity, greatly improving its anti-bacterial properties compared to simple CH or quaternary ammonium salts but also overcomes the limitation of its anti-bacterial and bactericidal being effects only under acidic conditions. This modification allows it to exhibit strong anti-bacterial effects under acidic, neutral, and alkaline conditions while retaining the other properties of CH30. Therefore, HACC was chosen as another monomer to form a physically crosslinked hydrogel system with improved stability and anti-bacterial properties (Fig. 1a). In this experiment, we used NxHy to name the hydrogels with different ratios of the two monomers, NAGA and HACC, where x and y represent the mass percentages (wt%, relative to the weight of deionized water) of NAGA and HACC, respectively.
a Design of conductive hydrogel materials with anti-bacterial efficacy with NAGA and HACC. b The drawing of the electrode support and mold assembly was created using Solidworks software (above) and the physical drawing of the hydrogel electrode (below). c SEM images of N40H3 and N40 are presented in (d)
Moreover, because the hydrogel itself has no movable charge or charge carrier, it is usually considered an electrical insulator. KCl was added to the hydrogel to obtain conductivity. The movement of ionic salts in the hydrogel matrix, activated by an electric field, results in an ionic conductive hydrogel that maintains its original mechanical flexibility and optical transparency while exhibiting high ionic conductivity31. To improve the moisturizing properties of the hydrogels, we added glycerol as a humectant to prevent water loss and compared the effects of adding lipidure-51, glycerol alone, or a mixture of both on the moisturizing properties of the hydrogels. Finally, APS was added as the coagulant promoter. After UV irradiation and heat-curing, conductive hydrogels with antibacterial properties were obtained.
According to the ergonomic design, we used Solidworks drawing software to design the support and mold as shown in Fig. 1b. The hard circular support was assembled with a relatively soft four-claw support structure, which allowed it to firmly hold the hydrogel and prevented it from falling off during use. The hydrogel prepolymer was poured into a semi-circular mold and cured to obtain hydrogel electrodes. The semi-circular design can increase the contact area between the electrodes and scalp hair, thereby improving signal transmission and enhancing wearer comfort.
The detailed steps for the electrode fabrication were as follows: the metal clasp and Ag/AgCl electrodes were assembled on top of the 3D-printed circular support using a clasp-making tool, and then placed in a 3D-printed semi-circular mold. The hydrogel prepolymer was poured through four small holes in the circular support to fill the mold. After the curing process was completed, the hydrogel electrode was gently peeled off from the mold to obtain a transparent hydrogel electrode, as shown. The conductive hydrogel was in contact with the Ag/AgCl electrode on one side and in direct contact with the scalp on the other side. The detected EEG signal could be transmitted from the epidermis to the Ag/AgCl electrode through the movement of Cl−.
Microstructure of hydrogel
The surface morphology and microstructure of the hydrogels were further observed by SEM, as shown in Fig. 1c, d, respectively. It can be seen that the hydrogel has a smooth cross-section and an abundant porous texture was found on the sides. This is because the concentrated aqueous solution of PNAGA forms a high-strength supramolecular polymer network structure, with many microscopic pores. Meanwhile, a large number of tens of microns of pores existed on the sides of the hydrogel samples, and the pores were uniformly distributed on the surface of the material. These pores provide sufficient storage space for water and glycerol, resulting in hydrogels with a relatively high water content.
Measurement of hydrogel mechanical properties and moisture retention properties
The mechanical properties of hydrogels are an important requirement for EEG electrodes, and compressive strength is an important parameter for evaluating these properties. To investigate the effects of different monomer ratios on the mechanical properties of the hydrogels, we conducted a series of compression tests. Figure 2a shows the compressive modulus of the hydrogels with different proportions of NAGA and HACC. It can be seen that both the compressive strength and modulus increase with the increase of NAGA content, which is attributed to the enhancement of stiffness due to the enhancement of the density of hydrogen-bonded supramolecular interactions32. To improve the wearing comfort of the subjects while ensuring that the hydrogel electrodes have good mechanical properties, we chose a hydrogel with a NAGA mass percentage of 40 wt% for subsequent experiments (65 kPa). Figure 2b shows the stress–strain curve of hydrogel compression of N40Hy. The HACC content has a smaller effect on the mechanical properties of the hydrogel, and we will determine the optimal proportion of HACC through subsequent experiments.
a Compression modulus of hydrogels with varying NAGA and HACC ratios. b The compressive stress-strain curves of hydrogels with 40 wt% NAGA and varying ratios of HACC. c Stress–strain curves and compression modulus of hydrogels with different KCl contents. d The original shape of the hydrogel and the shape after 500 compressions. e Stress–strain curves and compression modulus of hydrogels for 500 compression cycles. f Stress–strain curves and compression modulus of hydrogels for 10,000 compression cycles. g Changes in the appearance of hydrogels with different humectants added for 24 h under ventilated conditions. h Changes in water retention of hydrogels over 12 h under ventilated conditions. i Changes in long-term water retention of hydrogels under ventilated conditions
Next, we verified the effect of KCl concentration on the mechanical properties of the hydrogels (Fig. 2c). The compression modulus of the hydrogels gradually decreased with increasing KCl content, which may be attributed to the Hofmeister effect, that is ion-specific effect. Different ions affect the hydrated water molecules around the hydrophilic functional groups on the hydrophobic chain. Some anions polarize hydrated water molecules and destabilize the hydrogen bonds between the polymer and its hydrated water. Consequently, high concentrations of KCl can degrade the mechanical properties of hydrogels6.
The anti-fatigue and self-recovery properties of hydrogel electrodes are crucial for repeated use. To investigate this, we conducted 500 compression cycle tests on a hydrogel. Figure 2d compares the shape and height of the hydrogel before compression and after 500 cycles. The curves for the selected cycles (1st, 100th, 200th, 300th, 400th, and 500th) nearly overlap, showing no significant hysteresis (Fig. 2e). There was no fracture of the hydrogel during compression, and the height remained relatively unchanged, indicating excellent fatigue resistance and self-recovery properties. Next, we performed 10,000 compression cycle experiments on the hydrogel, and the experimental results (Fig. 2f) showed that there was no obvious hysteresis in the stress–strain curves of the compression cycles, which fully proved that the hydrogel could maintain good elasticity recovery ability after a large number of repetitive compressions, and provided strong data support for the stability and reliability of the hydrogel in practical applications, especially as the EEG electrodes need to withstand repetitively. The above results demonstrate that the hydrogel has good mechanical properties, can be reused in practical applications, is not easily damaged, and has great potential for use in portable EEG electrodes.
The moisturizing ability of the hydrogel has a direct impact on the service life of the electrodes, as the hydrogel becomes fragile after losing water, and the skin–electrode contact impedance becomes unstable. Therefore, we needed to find a suitable moisturizing agent to be added to the hydrogel. For this purpose, we compared the effects of two commonly used moisturizing agents: glycerol and lipidure-51, on the moisturizing ability of the hydrogel. Glycerol, or propanetriol, has three hydroxyl groups in its structural formula. The greater the density of the distribution of hydroxyl groups throughout the molecule, the greater the hygroscopicity. Glycerol’s structure is simple, its three hydroxyl groups are completely exposed, making it easier to combine with water, thus propanetriol providing very strong water absorbency33. Lipidure-51 is a biocompatible ingredient designed to mimic cell membranes and is a highly effective, gentle moisturizing factor with excellent water-locking and hydrating properties. Figure 2g shows the morphological changes of three hydrogels after 24 h of placement in a ventilated environment: one without a humectant, one with only glycerol as a humectant, and one with glycerol and lipidure-51 as humectants. It can be seen that the hydrogel with only glycerol (middle) shows the least change in morphology and does not exhibit significant water loss. In contrast, the hydrogel without any humectant (far left) has completely dried out after 24 h.
To systematically explore the moisture retention ability of hydrogels, we defined the water retention rate of hydrogels by placing hydrogels with different glycerol contents and lipidure-51 contents in a ventilated environment and recording W0 and Wt, respectively. As shown in Fig. 2h, i, the dehydration rate of the hydrogel gradually decreased with increasing glycerol content, indicating that the moisture retention ability of the hydrogel was improved, which is consistent with the above experimental results. The water retention of the hydrogel with only glycerol as the humectant was the highest, remaining above 75% for 6 weeks in room-temperature ventilation. This increase in water retention could be attributed to the hygroscopic capacity of glycerol. These results show that our hydrogel electrodes can be stored in air at room temperature for a long time without the need for special environments, which facilitates their use of hydrogel electrodes in subsequent monitoring.
Characterization of electrical properties
Electrical properties are a key feature of EEG electrodes used for signal recording. Here, we employed the four-electrode method and measured the contact impedance of hydrogel electrodes using an animal skin model (pig skin). A schematic of the impedance setup is shown in Fig. 3a. First, we examined the effect of different KCl concentrations on the contact impedance of hydrogel electrodes (Fig. 3b). The contact impedance of the electrodes gradually decreased with increasing KCl concentration, which was attributed to the fact that a high concentration of ionic salts increases the efficiency of ions moving between the skin and the electrodes, thereby decreasing the impedance. At a KCl concentration of 0.75 mol/L, the decreasing trend of the contact impedance slowed down to ∼300 Ω. Considering that too high a salt ion concentration reduces the mechanical properties of the hydrogel, we chose a salt concentration of 0.75 mol/L KCl for subsequent experiments. The contact impedance of wet electrodes in general EEG acquisition is <5 kΩ, which allows the acquisition of high-quality EEG, EMG, and other bioelectric signals for wide usage in scientific, cognitive, and clinical research11. Next, we examined the contact impedance changes of the wet and hydrogel electrodes for 12 h at room temperature (Fig. 3c). The wet electrodes initially had a contact impedance of around 250 Ω, but as time passed, it rapidly lost moisture, and the contact impedance gradually increased. After 6 h it completely dried out and the impedance became infinitely large (>30 kΩ), making it impossible to detect its electrical signal. In contrast, the contact impedance of the hydrogel electrodes did not significantly increase over time. The impedance of the hydrogel electrodes remained below 400 Ω for 12 h of operating voltage and gradually stabilized over time, which was attributed to the fact that the water content in the hydrogel reached a steady state. Thus, the wetting effect on the stratum corneum was almost constant, suggesting that the hydrogel electrode has certain advantages in the detection of EEG signals over a long period of time.
a Schematic diagram of the principle of skin–electrode contact impedance measurement using the four-electrode method. b Contact impedance of hydrogel electrodes with different KCl concentrations. c The contact impedance of the wet and hydrogel electrodes was measured for 12 h consecutively at room temperature. d Electrical impedance of hydrogels with different KCl contents over a wide frequency range. e Electrical impedance and phase angle of hydrogels at different frequencies. f CV cycling curves (1000 cycles) for an exposed area of 1.58 cm2. g Calculated CSC during different CV cycles. h CIC curves at the 1st, 1000th, 5000th, and 10,000th cycles of hydrogel electrodes. i CIC curves of anti-bacterial conductive hydrogel interfaces with an exposed area of 1.58 cm2. j Conductivity of hydrogels with different KCl contents at different frequencies
We also tested the EIS of the hydrogels using an electrochemical workstation with a frequency range of 0.1 Hz–0.1 MHz and a signal voltage setting of 5 mV. As shown in Fig. 3d, we first tested the contact impedance of hydrogels with different KCl contents in different frequency ranges. Typically, the impedance decreases with increasing test frequency, and a high concentration of KCl can effectively reduce the impedance of hydrogels. Figure 3e shows the electrical impedance and phase angle of the hydrogel at different frequencies. It can be seen that the phase of the hydrogel does not show a particularly significant hysteresis, indicating that the hydrogel electrode can collect bioelectric signals stably.
We used CV to measure the electrical stability of the hydrogel electrodes. We performed 1000 CV cycle tests (Fig. 3f), and the prepared hydrogel (0.77 mC cm−2), had a higher CSC than that of the commercial electrode (0.0019 mC cm−2), and the CSC, although slightly changed after 1000 CV cycles, the amount of change was <10% (Fig. 3g). We further characterized the CIC of the anti-bacterial conductive hydrogel interface (Fig. 3h, i), and from the experimental results, we can see that the hydrogel electrode maintains excellent charge injection and storage stability, and the out-of-plane impedance of the hydrogel interface exhibits negligible hysteresis and fluctuation under bidirectional pulse stimulation. Subsequently, the ionic conductivities of the hydrogels. A hydrogel with a diameter of 10 mm and a height of 3 mm was tightly sandwiched between two copper sheets, and EIS was measured in the potentiation control mode. The experimental results are shown in Fig. 3j. The ionic conductivity of the hydrogel increased with increasing potential frequency and KCl concentration, reaching around 0.39 mS cm−1 at EEG-related frequencies (0.5–50 Hz)34, which allowed for continuous and effective collection of EEG signals.
Characterization of anti-bacterial properties
The surfaces of typical hydrogel electrodes often carry bacteria from the skin, hair, or air of the subject after use, which can lead to mold growth during recycling and storage. This makes the electrodes unusable, resulting in significant waste and higher costs. Additionally, if a subject has a wound on the scalp, it increases the risk of infection. Therefore, the development of a hydrogel electrode with anti-bacterial properties is essential for long-term repeated EEG acquisition. The anti-bacterial mechanism of HACC is due to the large number of positive charges on the surface of its molecules, which interact with the negative charges on the microbial cell membranes. This interaction alters the permeability of cell membranes, leading to the leakage of bacterial proteins and other cellular components, thereby producing anti-bacterial effects35. To test the anti-bacterial properties of hydrogel electrodes, we selected Gram-negative bacteria represented by E. coli and Gram-positive bacteria represented by S. epidermidis. S. epidermidis is a bacterium that is widespread on human skin36. Overgrowth of S. epidermidis can lead to serious skin diseases, including atopic dermatitis (AD)37, Netherton’s syndrome (NS)38, and seborrheic dermatitis/dandruff of the scalp39.
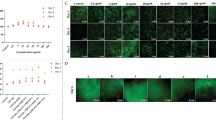
First, the size of the anti-bacterial circle of the hydrogel against the two bacteria was determined using the coated plate method (Fig. 4a). There was no visible anti-bacterial zone around the hydrogel in the control group without added HACC. In contrast, obvious anti-bacterial zones appeared around all HACC-added hydrogels, and the anti-bacterial effect became more pronounced with increasing HACC concentration. The diameter of the anti-bacterial circle was the largest at HACC content of 3 wt%. The diameters of the zone of inhibition of N40H3 hydrogels against E. coli and S. epidermidis were 20.75 ± 1.08 and 15.17 ± 0.54 mm, respectively (Fig. 4b, c). The anti-bacterial activity of the N40H3 hydrogel was further confirmed by the OD counting method, as shown in Fig. 4d, f. The control hydrogel showed no anti-bacterial effect against E. coli or S. epidermidis, with a gradual increase in UV absorption at 600 nm. In contrast, the N40H3 hydrogel showed significant anti-bacterial activity, which increased significantly over time. The N40H3 hydrogel inhibited more than 80% of E. coli after 10 h and more than 95% of S. epidermidis after 7.5 h. Additionally, the bacterial suspensions were aspirated at each time point, diluted, and spread on LB and NA agar plates. The colony growth status after 24 h in the incubator is shown in Fig. 4e, g. These results were consistent with the OD measurements: the plates of the control group showed a large number of colonies, while the number of colonies on the LB plates with the N40H3 hydrogel gradually decreased, and almost no colonies appeared on the NA plates. These results demonstrated that the addition of HACC endowed the hydrogel with strong anti-bacterial properties.
a Anti-bacterial inhibition of E. coli on LB agar plates and S. epidermidis on NA agar plates by hydrogels with different HACC contents. b Diameter of the anti-bacterial circle in which hydrogels inhibit the growth of E. coli. c Diameter of the anti-bacterial circle of hydrogel inhibiting the growth of S. epidermidis. d Changes in OD values of hydrogels acting in E. coli suspensions for 10 h. e Results of coated plates after dilution of hydrogel/E. coli suspension. f Changes in OD values of hydrogels acting in S. epidermidis suspensions for 24 h. g Results of plate coating after dilution of hydrogel/S. epidermidis suspension
We invited two male subjects to wear the control and N40H3 hydrogel electrodes for three consecutive days to further examine the compression resistance and anti-bacterial properties of the hydrogel electrodes after use. Each subject wore 3 control and 3 N40H3 hydrogel electrodes on the occipital region of the hindbrain area and did not experience any discomfort during the 3-day period. As shown in Fig. 5a, the height of the N40H3 hydrogel electrode remained comparable to its original height after 3 days of use, with no significant changes, breaks, or cracks on the side in contact with the subject’s hair. It is further illustrated that hydrogel electrodes have good mechanical strength that is compatible with human tissue and superior fatigue resistance. The two groups of hydrogels were kept at room temperature, and after 1-month, visible colonies appeared on the surface of the control hydrogel (Fig. 5b), whereas this did not occur with the experimental hydrogel. Next, we tested the anti-bacterial properties of the hydrogels on the surface by placing the two hydrogel electrodes in LB and NA liquid media, respectively. Figure 5c shows the two groups of hydrogels after 24 h in the medium, clearly indicating that both mediums containing the control hydrogel became very turbid, whereas the medium containing the N40H3 hydrogel remained clear. Subsequently, we measured the OD values of the hydrogel suspensions at various time points (Fig. 5d). No change was observed in the OD value of the medium in which the N40H3 hydrogels were placed, further demonstrating the superior anti-bacterial properties of the hydrogels. These results suggest that hydrogel electrodes can be recovered and reused in daily applications.
a Comparison of the appearance of hydrogel electrodes with the original hydrogel electrodes after being worn by the subjects for 3 days. b Appearance of bacterial colonies on the surfaces and inside of the worn hydrogel electrodes after being stored for 1 month at room temperature. c Results of the action of the used control and N40H3 hydrogel electrodes in the LB/NA liquid medium after 24 h. d Changes in OD of the hydrogel electrode suspension in the LB/NA liquid medium with different HACC contents. e Cell proliferation rate of hydrogels with different HACC contents after 3 days of immersion in complete DMEM. f Thickness of granulation tissue after H&E staining of skin tissue for hydrogel action. g Images of hydrogels applied to the dorsal skin of female and male SD rats 1–7 days after application. h Results of H&E staining of skin tissue to which hydrogel was applied (green arrows represent dermis, blue arrows represent nuclei)
Biocompatibility and biosafety
When hydrogel electrodes are used to collect EEG signals, the hydrogel comes into direct contact with the skin of the subject. Therefore, it is important that hydrogel electrodes exhibit good biocompatibility. To confirm this, we performed cytotoxicity tests and skin irritation tests on the hydrogel electrodes.
First, we assessed the biocompatibility of the hydrogels by determining their cellular proliferation rate using CCK-8. Figure 5e shows the results of 3T3 cells cultured in extracts of hydrogels with different concentrations of HACC immersed in complete DMEM for 3 days. The cell proliferation rate of N40H3 cells was above 90%, indicating good biocompatibility. In contrast, the cell proliferation rate of hydrogels with HACC mass percentages above 4 wt% decreased more significantly. Therefore, we selected a hydrogel electrode with a monomer ratio of N40H3.
We examined the biosafety of the hydrogel in vitro, using SD rats as test animals, with both female and male rats tested for allergic and inflammatory reactions to the hydrogels. Figure 5g shows images of the hydrogels applied to the bare back skin of rats after days 1, 3, 5, and 7. Compared with the shaved blank group, no redness, swelling, inflammation, or exudation appeared on the back skin of both female and male rats during the 7 days of hydrogel application. This indicates that the hydrogel can be in contact with the skin for a long period of time without causing serious allergic reactions.
To further assess the skin irritation caused by the hydrogel, we collected rat skin tissues with hydrogel applied at various time points, embedded them in paraffin wax, and stained them with H&E. The results are shown in Fig. 5h, the upper row shows the straining result of female rat skin tissue (scale bar: 20 μm), and the lower row shows the straining result of male rat skin tissue (scale bar: 50 μm). In comparison with the blank group, none of the experimental groups showed inflammatory signals, such as hemocytes and neutrophils, nor significant thickening of the epidermis at the 7-day time point. Next, we measured the thickness of the granulation tissue at each time point for quantitative evaluation (Fig. 5f). The results showed no significant difference between the experimental and control groups (p > 0.05). These findings confirm that hydrogel electrodes have good biocompatibility and biosafety.
Acquisition of EEG signals
We measured the real-time contact impedance of the wet electrodes and hydrogel electrodes for 12 h consecutively, using the wet electrode as the “gold standard” for comparison. Figure 6a, b show the reference and working electrodes, respectively, connected to the EEG cap worn by the subject during the experiment. We chose GND and CPz as the reference electrodes and simultaneously measured the real-time contact impedance of wet electrodes and the hydrogel electrodes in regions with normal hair (P3, P4, and O2) and regions with fewer hairs (Fp1 and F8). The results are shown in Fig. 6c, d. The contact impedance of the wet electrodes gradually increased owing severe dehydration after 6 h in the normal hair region, and the less hairy region was completely dry after 7 h, and no EEG signal could be detected. In contrast, the contact impedance of the hydrogel electrodes remained stable for 12 h consecutively, showing no significant fluctuations and staying below 100 kΩ, thus enabling long-term acquisition of EEG signals.
a Pictures of subjects measuring real-time contact impedance in a sonic darkroom. b Pictures of reference and working electrodes used for EEG measurements (GND and CPz are the reference electrodes, and the rest are the working electrodes). c Real-time contact impedance of the wet electrodes and the hydrogel electrodes in the area of less hair. d Real-time contact impedance of the wet electrodes and the water gel electrodes in areas with normal hair. e Schematic of N170 test. f Signal-to-noise ratios of dry, wet, and hydrogel electrodes. g Generation of N170 waves at O1 and O2 using dry, wet, and hydrogel electrodes. h Generation of N170 waves at O1 using hydrogel electrodes for 21 days (worn for 2 h per day). i Variation of signal-to-noise ratios of hydrogel electrodes for 21 consecutive days
EEG acquisition by measuring event-related potentials (ERPs) can be effectively used to assess brain function. ERPs provide a practical way to identify the efficacy of non-invasive BCI electrodes40. Figure 6e illustrates a schematic of how the subjects responded to the prompts in the N170 test. In the acquisition of EEG signals, the functional areas of the brain in which the signals were acquired for the emotion-evoking test were the left occipital lobe (O1) and the right occipital lobe (O2) of the occipital lobe of the hindbrain. The results of the experiment are shown in Fig. 6g. The hydrogel electrodes showed waveforms similar to those of the wet electrodes in the O1 and O2 regions, displaying the expected negative peaks around 170 ms, indicating that the hydrogel electrodes successfully detected ERPs. However, the waveforms of the dry electrodes were significantly different from those of the wet and hydrogel electrodes, with no significant negative peaks within the time window of N170. At the same time, we calculated the signal-to-noise ratios of the dry, wet, and hydrogel electrodes (Fig. 6f), which showed that the signal-to-noise ratios of the hydrogel electrodes (20.02 dB) were similar to those of the ‘gold-standard’ wet electrodes, and were much higher than those of the dry electrodes. The above results confirm that hydrogel electrodes can detect EEG signals at the microvolt level in subjects and allow prolonged monitoring of EEG signals. By making the volunteers wear the electrodes for 2 h a day for 21 consecutive days to collect N170 signals, the experimental results showed that the hydrogel electrodes were able to stably monitor the expected signal waveforms. Moreover, the signal-to-noise ratio of the hydrogel electrode did not show any significant decrease during 21 consecutive days of signal acquisition, which improved the long-term durability and service life of the electrodes.
Conclusion
In this study, we present a semi-dry, durable, conductive hydrogel electrode featuring antibacterial properties, designed for easy installation and convenient use. The electrode demonstrated superior antibacterial properties, enabling recycling and reuse while consistently acquiring high-quality EEG signals over extended periods of time. The hydrogel exhibited robust mechanical properties capable of maintaining its original height even after 500 compression cycles, ensuring wearer comfort. Moreover, the conductive and water-retaining properties of the hydrogel effectively keep the skin–electrode contact impedance in a reasonably low range. Most importantly, our hydrogel effectively inhibited the growth of both Gram-negative (E. coli) and Gram-positive (S. epidermidis) bacteria, facilitating electrode recycling and minimizing the risk of bacterial transmission. Cell proliferation and skin irritation tests confirmed the biocompatibility of the hydrogel electrodes, ensuring no harm will come to the wearer’s skin. N170 trials on human volunteers demonstrate the hydrogel electrodes’ ability to capture EEG signals at microvolt levels with high performance. Additionally, the results indicate that the SNR of the hydrogel electrodes is comparable to that of the “gold standard” wet electrodes, which is significantly higher than that of dry electrodes. During 21 consecutive days of monitoring, the hydrogel electrodes consistently acquired the expected signal waveforms and no significant decrease in the SNR of the hydrogel electrodes was observed. Compared with traditional Ag/AgCl wet electrodes, our hydrogel electrodes offer easier installation, eliminate the need for post-use cleaning, and can continuously record EEG for over 12 h. In contrast, wet electrodes lose functionality after approximately 5 h owing to dehydration. In summary, the developed conductive hydrogel electrodes with antibacterial properties in this study present a user-friendly solution with significant potential for long-term EEG recordings in daily life applications.
References
Rao, R. P. Towards neural co-processors for the brain: combining decoding and encoding in brain–computer interfaces. Curr. Opin. Neurobiol. 55, 142–151 (2019).
Al-Saegh, A., Dawwd, S. A. & Abdul-Jabbar, J. M. Deep learning for motor imagery EEG-based classification: a review. Biomed. Signal Process. Control 63, 102172 (2021).
Abdulkader, S. N., Atia, A. & Mostafa, M. M. Brain computer interfacing: applications and challenges. Egypt. Inform. J. 16, 213–230 (2015).
Mridha, M. F., Das, S. C., Kabir, M. M., Lima, A. A., Islam, M. R. & Watanobe, Y. Brain–computer interface: advancement and challenges. Sensors (Basel, Switzerland) 21, 5746–5758 (2021).
Bulut, S. The brain–computer interface. Int. Conf. Tech. Technol. Educ. 7, 133–138 (2019).
Wu, Y. et al. Hofmeister effect and electrostatic interaction enhanced ionic conductive organohydrogels for electronic applications. Adv. Funct. Mater. 32, 2110859 (2022).
Liu, Y. et al. Nanomaterial-based microelectrode arrays for in vitro bidirectional brain–computer interfaces: a review. Microsyst. Nanoeng. 9, 13–31 (2023).
Cao, H. et al. Research advances in non-invasive brain–computer interface control strategies. J. Biomed. Eng. 39, 1033–1040 (2022).
Afsharipour, B., Soedirdjo, S. & Merletti, R. Two-dimensional surface EMG: the effects of electrode size, interelectrode distance and image truncation. Biomed. Signal Process. Control 49, 298–307 (2019).
Yuan, H. et al. State of the art of non-invasive electrode materials for brain–computer interface. Micromachines 12, 1521–1534 (2021).
Li, G., Wang, S. & Duan, Y. Y. Towards conductive-gel-free electrodes: understanding the wet electrode, semi-dry electrode and dry electrode–skin interface impedance using electrochemical impedance spectroscopy fitting. Sens. Actuators B Chem. 277, 250–260 (2018).
He, G., Dong, X. & Qi, M. From the perspective of material science: a review of flexible electrodes for brain–computer interface. Mater. Res. Express 7, 102001 (2020).
Searle, A. & Kirkup, L. A direct comparison of wet, dry and insulating bioelectric recording electrodes. Physiol. Meas. 21, 271–283 (2000).
Niu, X., Gao, X., Liu, Y. & Liu, H. Surface bioelectric dry electrodes: a review. Measurement 183, 109774 (2021).
Lai, H. et al. Temperature‐triggered adhesive bioelectric electrodes with long‐term dynamic stability and reusability. Adv. Sci. 10, 2300793 (2023).
Vasconcelos, B., Fiedler, P., Machts, R., Haueisen, J. & Fonseca, C. The arch electrode: a novel dry electrode concept for improved wearing comfort. Front. Neurosci. 15, 748100 (2021).
Li, G., Wu, J., Xia, Y., He, Q. & Jin, H. Review of semi-dry electrodes for EEG recording. J. Neural Eng. 17, 51004 (2020).
Su, M. et al. Current state of knowledge on intelligent-response biological and other macromolecular hydrogels in biomedical engineering: a review. Int. J. Biol. Macromol. 227, 472–492 (2023).
Zhu, T. et al. A semi-interpenetrating network ionic hydrogel for strain sensing with high sensitivity, large strain range, and stable cycle performance. Chem. Eng. J. 385, 123912 (2020).
Han, Q. et al. Hydrogel nanoarchitectonics of a flexible and self-adhesive electrode for long-term wireless electroencephalogram recording and high-accuracy sustained attention evaluation. Adv. Mater. 35, 2209606 (2023).
Jakab, K. et al. EEG sensor system development consisting of solid polyvinyl alcohol–glycerol–NaCl contact gel and 3D-printed, silver-coated polylactic acid electrode for potential brain–computer interface use. Mater. Today Chem. 26, 101085 (2022).
Xue, H. et al. Hydrogel electrodes with conductive and substrate-adhesive layers for noninvasive long-term EEG acquisition. Microsyst. Nanoeng. 9, 79 (2023).
Cao, H., Duan, L., Zhang, Y., Cao, J. & Zhang, K. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct. Target. Ther. 6, 426–434 (2021).
Wang, J. et al. Sponge inspired flexible, antibacterial aerogel electrode with long-term high-quality electrophysiological signal recording for human–machine interface. Adv. Funct. Mater. 34, 2309704 (2024).
Lin, X. et al. Ultralow fouling and functionalizable surface chemistry based on zwitterionic carboxybetaine random copolymers. Langmuir 35, 1544–1551 (2019).
Lan, L. et al. Skin-inspired all-natural biogel for bioadhesive interface. Adv. Mater. 36, 2401151 (2024).
Thigpen, N. N., Kappenman, E. S. & Keil, A. Assessing the internal consistency of the event-related potential: an example analysis. Psychophysiology 54, 123–138 (2017).
Xu, Z. & Liu, W. Poly(N-acryloyl glycinamide): a fascinating polymer that exhibits a range of properties from ucst to high-strength hydrogels. Chem. Commun. 54, 10540–10553 (2018).
Yang, J. et al. Advanced applications of chitosan-based hydrogels: from biosensors to intelligent food packaging system. Trends Food Sci. Technol. 110, 822–832 (2021).
Andreica, B., Cheng, X. & Marin, L. Quaternary ammonium salts of chitosan. A critical overview on the synthesis and properties generated by quaternization. Eur. Polym. J. 139, 110016 (2020).
Liu, X., Liu, J., Lin, S. & Zhao, X. Hydrogel machines. Mater. Today 36, 102–124 (2020).
Dai, X. et al. A mechanically strong, highly stable, thermoplastic, and self-healable supramolecular polymer hydrogel. Adv. Mater. 27, 3566–3571 (2015).
Ben, Z. Y., Samsudin, H. & Yhaya, M. F. Glycerol: its properties, polymer synthesis, and applications in starch based films. Eur. Polym. J. 175, 111377 (2022).
Yuan, H., Zotev, V., Phillips, R. & Bodurka, J. Correlated slow fluctuations in respiration, EEG, and bold fMRI. Neuroimage 79, 81–93 (2013).
Sajomsang, W., Gonil, P. & Tantayanon, S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: preparation and characterization. Int. J. Biol. Macromol. 44, 419–427 (2009).
Severn, M. M. & Horswill, A. R. Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat. Rev. Microbiol. 21, 97–111 (2023).
Byrd, A. L. et al. Staphylococcus Aureus and Staphylococcus Epidermidis strain diversity underlying pediatric atopic dermatitis. Sci. Transl. Med. 9, eaal4651 (2017).
Williams, M. R. et al. Interplay of staphylococcal and host proteases promotes skin barrier disruption in Netherton syndrome. Cell Rep. 30, 2923–2933 (2020).
Clavaud, C. et al. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS ONE 8, e58203 (2013).
Toyama, S., Takano, K. & Kansaku, K. A non-adhesive solid-gel electrode for a non-invasive brain–machine interface. Front. Neurol. 3, 114–122 (2012).
Acknowledgements
This work is supported by the Ministry of Science and Technology of China (STI 2030—Major Projects 10700, No. 2022 ZD0210700).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
J.L. and D.W. have filed a patent for the development of the described hydrogel electrodes for EEG acquisition in noninvasive BCI applications.
Ethics
This study was approved by the Biological and Medical Ethics Committee of Dalian University of Technology (approval number: DUTSBE250228-09).
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, D., Xue, H., Xia, L. et al. A tough semi-dry hydrogel electrode with anti-bacterial properties for long-term repeatable non-invasive EEG acquisition. Microsyst Nanoeng 11, 105 (2025). https://doi.org/10.1038/s41378-025-00908-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41378-025-00908-4