Abstract
Mucinous colorectal adenocarcinoma (CRC) is conventionally defined by extracellular mucin comprising >50% of the tumour area, while tumours with ≤50% mucin are designated as having a mucinous component. However, these definitions are largely arbitrary and comparisons of clinico-molecular features and outcomes by proportion of mucinous component are limited. A cohort of 1643 patients with stage II/III cancer was examined for tumour mucinous component, DNA mismatch repair (MMR) status, BRAF mutation and tumour infiltrating lymphocytes (TILs). Tumours with ≤50% mucinous component exhibited similar characteristics as mucinous tumours, including association with female gender, proximal ___location, high grade, TIL-high, defective MMR (dMMR) and BRAF mutation. Proportion of mucinous component did not stratify disease-free survival (DFS). In univariate analysis dMMR status, but not histological grade, stratified survival for mucinous and mucinous component tumours; however, in multivariate analysis dMMR status was not an independent predictor. BRAF mutation prognostic value depended on mucinous differentiation and MMR status, with poor prognosis limited to non-mucinous pMMR tumours (HR 2.61, 95% CI 1.69–4.03; p < 0.001). TIL status was a strong independent predictor of DFS in mucinous/mucinous component tumours (HR 0.40, 95% CI 0.23–0.67; p < 0.001), and a superior predictor of prognosis compared with histological grade, MMR and BRAF mutation. Mucinous component and mucinous stage II/III CRCs exhibit clinico-molecular resemblances, with histological grade and BRAF mutation lacking prognostic value. Prognosis for these tumours was instead strongly associated with TIL status, with the most favourable outcomes in TIL-high dMMR tumours, whilst TIL-low tumours had poor outcomes irrespective of MMR status.
Similar content being viewed by others
Introduction
Mucinous colorectal adenocarcinoma is conventionally defined as colorectal cancer (CRC) in which more than 50% of the lesion is composed of pools of extracellular mucin [1]. Mucinous tumours exhibit variation in geographical distribution, accounting for ~5% of CRC in studies from Asian countries [2,3,4], and 10–20% in studies from Western countries [5,6,7,8]. Compared with non-mucinous adenocarcinoma, mucinous tumours are more common in the proximal colon and exhibit specific molecular features including DNA mismatch repair deficiency (dMMR) [8,9,10,11,12,13,14,15,16], BRAF mutation [10, 13,14,15,16] and a high-frequency of CpG island methylator phenotype (CIMP-high) [10, 13, 16]. Mucinous CRC has further been negatively associated with p53 overexpression [12, 16,17,18]. The definition of mucinous CRC based on >50% mucin component, while traditional, is largely arbitrary. Limited data exist comparing the characteristics and survival of patients with mucinous tumours to those with tumours with ≤50% mucinous component [8, 11, 13, 17,18,19,20,21,22].
There are conflicting studies regarding the prognostic impact of mucinous differentiation in CRC. While some studies have reported that mucinous tumours have a worse prognosis than non-mucinous tumours [2, 6, 12, 19, 23], others have found no difference [4, 5, 7, 11, 13, 24]. The lack of consensus may be attributable to the diversity of patients included, small cohort size because of the rarity of the mucinous subtype or limited patient follow-up. In addition, many studies have been limited by their inability to control for key molecular features of CRC, such as dMMR status and BRAF mutation, which are potential confounders. In early-stage CRCs, dMMR status has been associated with better survival [25, 26], while BRAF mutation has been associated with particularly poor survival in DNA mismatch repair proficient (pMMR) tumours [27,28,29]. Moreover, the prognostic significance of ≤50% tumour mucinous component remains unclear.
Histologic grading of mucinous CRC is challenging. Conventionally, colorectal adenocarcinoma is graded based on the degree of glandular differentiation, with cases exhibiting ≤50% gland formation being considered as high grade [1]. The applicability of this grading scheme to mucinous carcinomas is contentious. World Health Organisation (WHO) 4th Edition guidelines (2010) recommended that mucinous CRCs should instead be graded based on MMR status, with pMMR tumours considered as high grade [1]. However, evidence that mucinous tumours can be graded exclusively based on MMR status was derived from smaller studies or subgroup analyses [11, 30, 31], and the WHO 5th Edition guidelines (2019) were revised to recommend that mucinous CRCs should be graded using the same histological criteria as conventional non-mucinous CRC [32]. Limited data exist regarding the prognostic value of histological grade and MMR status for tumours with a mucinous component [5, 12,13,14, 19].
Host immune response against tumour has emerged as a strong prognostic predictor for CRC. High density of tumour infiltrating lymphocytes (TILs) as determined by histopathological examination has been associated with lower rates of recurrence and longer patient survival in multiple studies [33,34,35,36]. Scoring systems such as global TIL density or assessments of T-lymphocyte populations have been proposed as prognostic classifiers [36,37,38,39]. Recent data suggest that combined consideration of TIL/MMR status provides a refined prognostic stratification for patients with stage II/III CRC [36, 39,40,41]. Specifically, TIL status appears to stratify both dMMR and pMMR tumours by outcome, with TIL-high tumours exhibiting significantly better outcomes. Whether TIL status refines prognostication for tumours with mucinous differentiation has not been evaluated.
The purpose of the present study was to evaluate the clinico-molecular features and oncological outcomes of CRC according to the proportion of mucinous component in a community-based cohort of 1643 patients with stage II/III disease. In particular, we evaluated the prognostic value of tumour classification by mucinous component and examined the changing WHO recommendations regarding whether mucinous CRCs should be graded on the basis of either dMMR status or glandular morphology. We explored prognostic interactions between clinico-molecular variables and tumour mucinous component and investigated the prognostic value of TIL status.
Materials and methods
Study population
A total of 1643 patients with resected stage II or III CRC were recruited at the Royal Melbourne Hospital, Western Hospital Footscray, Austin Health and St Vincent’s Hospital Sydney between 1999 and 2015. Signet-ring carcinoma or cases that had received neoadjuvant chemoradiation were excluded, as were individuals with hereditary CRC syndromes. Formalin-fixed paraffin-embedded tumour and matched normal tissue specimens were obtained at surgery. Patients were followed according to standard protocols (see Supplementary Methods). Clinicopathologic treatment and outcome data were either collected prospectively (63.5%, 1044 of 1643 patients) or retrospectively assembled from medical records (36.5%, 599 of 1643 patients). The tumour ___location was defined as proximal (caecum to splenic flexure) and distal (splenic flexure to rectum). Patient characteristics are summarised in Supplementary Table S1. All participants gave informed consent, and this study was approved by the medical ethics committees of all sites (WEHI HREC 12/19, Austin HREC 2013/05077).
Histopathological evaluation
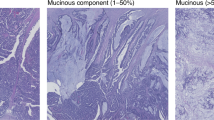
A central pathology review was performed for all cases based on haematoxylin and eosin (H&E)-stained tissue sections by a gastrointestinal pathologist (DSW) unaware of other clinical and molecular data. All original H&E slides of the primary tumours (≥3 sections per case) were available in 880 cases and 1 tumour slide was available for the remaining 763 cases. Mucinous differentiation was scored as present for either mucinous carcinoma (>50% extracellular mucin) or CRC with a mucinous component (1–50% mucin) (Supplementary Figure S1). A subset of 325 cases with multiple slides were reviewed by a second pathologist (EA or MN). Discordant cases were resolved by a further two pathologists (MN or EA and DKN). The concordance for assessing proportion of mucinous component (trichotomized as 0 vs. 1–50 vs. >50 %) was good (DSW vs. MN linear weighted kappa 0.80, 95% CI 0.73–0.87, DSW vs. EA linear weighted kappa 0.73, 95% CI 0.68–0.79). Tumour grade was categorised as high or low based upon WHO grading (≤50% vs. >50% glandular area) (Supplementary Figure S2, see Supplementary Methods).
Density of TILs for these tumours as scored using H&E-stained tissue sections has been reported previously [36]. Five consecutive random 40× fields of a BX51 microscope with 10× objective (Olympus) were counted for each H&E-stained tissue section, sampling representative different regions of each tumour (field diameter 0.55 mm, total area of five high powered fields (hpf) 1.188 mm2). For paucicellular tumours (e.g. low-grade mucinous adenocarcinomas comprising thin strips of epithelium at the periphery of the mucin pools), scoring was performed on tumour-enriched fields with the highest tumour cell density. Regions of precursor adenoma or necrosis were excluded from analysis, and only intraepithelial lymphocytes were counted, excluding those within the intervening stroma. The mean TIL per hpf for each tumour was calculated by dividing the total number of TILs by five. Tumours were classified into TIL-low (<2 TILs per hpf) and TIL-high (≥2 TILs per hpf) cases, according to a pre-determined cut-off value distinguishing dMMR and pMMR cases [36]. For tumours near the cut-point showing a heterogeneous distribution of TILs, scoring was commenced in a hot-spot region identified during low power screening, followed by random fields.
DNA mismatch repair status
MMR status for these tumours has been reported previously [36], assessed by either the Bethesda consensus panel of microsatellite markers or immunohistochemistry (IHC) (see Supplementary Methods).
BRAF mutation detection
BRAF (V600E) mutation status for these tumours has been reported previously [36], assessed either by Sanger sequencing (n = 1044) or by IHC (n = 599) (see Supplementary Methods). We have previously demonstrated high concordance of BRAF V600E IHC assessment with Sanger sequencing in a cohort of 477 CRCs, with sensitivity and specificity of 98.2% and 98.1%, respectively [42].
Statistical methods
Statistical analyses were conducted using the statistical computing software R (R Development Core Team, 2011). Interobserver reproducibility for determination of mucinous component between reviewers was assessed using Cohen’s kappa statistic. For univariate analyses, differences between groups were assessed using the Fisher’s exact test for categorical variables and the Student’s t test for continuous variables. Outcome analyses were conducted for 5-year disease-free survival (DFS). DFS was defined as time from surgery to the first confirmed relapse, with censoring done when a patient died or was alive without recurrence at last contact. Survival curves were generated according to the method of Kaplan and Meier. Cox proportional hazard models were used to assess the associations of tumour mucinous component with DFS in the context of patient clinico-molecular features and adjuvant treatment. Hazard ratios and 95% confidence intervals were calculated. Comparisons of Cox proportional hazard models with TIL status against models with tumour grade, MMR or BRAF mutation were made using the Aikake Information Criterion (AIC), a measure of how well a model fits a dataset, with a smaller AIC value indicating the better model. As described by Burnham and Anderson [43], an AIC difference >10, indicates that there is essentially no support for the model with the larger AIC of being as good a fit as the model with the lower AIC. The likelihood-ratio test, which assesses the goodness of fit of two competing models, was used to determine whether addition of TIL status improved models with tumour grade, MMR or BRAF status or vice versa. All statistical analyses were two-sided and considered significant if p < 0.050.
Results
Patient characteristics
Of the 1643 stage II/III patients, 51.6% (n = 847) were female, and the median age was 72 (range 22–99) years (Supplementary Table S1). 53.6% (879 of 1641) of cancers were from the proximal colon and 28.4% (467 of 1643) exhibited high grade. All patients underwent surgical resection with 42.3% (636 of 1503) receiving adjuvant chemotherapy. 22.1% (363 of 1643) of individuals experienced a disease recurrence. The median follow-up duration was 54 months.
Tumours were grouped based on mucinous component of 0%, 1–50%, and >50% into non-mucinous adenocarcinomas, adenocarcinomas with a mucinous component and mucinous adenocarcinomas, respectively. Tumour samples were analysed for MMR status and BRAF (V600E) mutations. 68.4% (1123 of 1643) of cancers were classified as non-mucinous, 12.2% (200 of 1643) as having a mucinous component, and 19.5% (320 of 1643) as mucinous. 26.2% (421 of 1604) of cancers were dMMR and 20.1% (321 of 1594) harboured a BRAF mutation. Tumour dMMR status displayed the anticipated strong direct association with BRAF mutation (OR = 15.3).
Relationship of tumour mucinous component with clinicopathologic and molecular characteristics
To evaluate whether tumours with a mucinous component should be clinically treated as being biologically distinct from conventional non-mucinous adenocarcinomas, we examined the relationship of mucinous differentiation with patient clinicopathologic and tumour molecular characteristics.
Tumours with a mucinous component tended to exhibit similar characteristics as mucinous carcinomas (Table 1). Compared with conventional non-mucinous adenocarcinomas, both mucinous tumours and tumours with a mucinous component were significantly associated with female gender, proximal ___location, high grade, dMMR, and BRAF mutation (p ≤ 0.037 for all comparisons). Patients with mucinous tumours and tumours with a mucinous component appeared marginally older than patients with non-mucinous tumours. The only differentiating characteristic between mucinous tumours and tumours with a mucinous component was tumour stage, with the former associated with stage II disease (p = 0.038); this association was also observed when comparing mucinous to non-mucinous tumours (p = 0.022).
Tumour mucinous component and disease-free survival
The prognostic value of tumour classification by mucinous component remains unclear. To examine the relationship of tumour mucinous component with outcome, we focused on DFS, an appropriate measure for CRC treated in the adjuvant setting [44].
Patients with non-mucinous, mucinous component and mucinous tumours had similar 5-year DFS in our cohort. In multivariate analysis adjusting for baseline clinico-molecular variables and treatment, hazard ratios for DFS for mucinous vs. non-mucinous, mucinous component vs. non-mucinous and mucinous component vs. mucinous tumours were 0.80 (95% CI 0.59–1.09; p = 0.163), 0.82 (95% CI 0.58–1.16; p = 0.260) and 1.02 (95% CI 0.67–1.54; p = 0.929), respectively (Table 2). Among clinico-molecular variables, tumour stage III, high grade and BRAF mutation were associated with reduced DFS while dMMR status and adjuvant treatment were associated with improved DFS (p ≤ 0.017 for all variables). Age at diagnosis, gender and tumour ___location were not associated with survival. In subgroup analyses stratified by dMMR status and tumour ___location, there were also no significant differences in DFS between tumours grouped by mucinous component (Supplementary Tables S2–3).
Evaluation of the WHO colorectal cancer grading system
Under the WHO 2010 guidelines it was recommended that mucinous colorectal carcinomas should be graded on the basis of MMR status [1]. Specifically, dMMR mucinous tumours should be considered as low grade, whereas pMMR tumours should be considered as high grade regardless of histological grading.
Consistent with these guidelines, we found that MMR status stratified mucinous carcinomas according to DFS in univariate analysis with 5-year DFS rates of 85.5% for dMMR and 73.3% for pMMR tumours (HR 0.52, 95% CI 0.30–0.93; p = 0.022; Fig. 1a), while histological grade did not stratify mucinous tumours by survival (Fig. 1d). As observed for clinico-molecular characteristics, tumours with a mucinous component again showed similar prognostic relationships as mucinous carcinomas, with 5-year DFS rates of 84.2% for dMMR and 69.1% for pMMR tumours (HR 0.45, 95% CI 0.24–0.86; p = 0.013; Fig. 1b), but similar outcomes by tumour grade (Fig. 1e). In support of the WHO 2010 tumour grading recommendations, the interaction between histological grade and mucinous component (mucinous/mucinous component vs. non-mucinous tumours) was significant in multivariate analysis adjusting for clinico-molecular features and treatment (pinteraction = 0.040), while no evidence for interaction was observed between MMR status and mucinous component (pinteraction = 0.448). However, in multivariate analyses of mucinous and non-mucinous tumours, dMMR status was not an independent prognostic predictor (Table 3A–C). This latter result is consistent with the recommendation in the WHO 2019 guidelines [32], but our findings do not support the suggestion that conventional histological grading should also be applied to mucinous tumours. In contrast, both tumour grade (HR 1.91, 95% CI 1.44–2.53; p < 0.001) and MMR status (HR 0.49, 95% CI 0.31–0.76; p = 0.001) were significant independent prognostic variables in non-mucinous tumours in multivariate analysis (Fig. 1c, f, Table 3D).
Patients are grouped according to tumour (a–c) MMR status and (d–f) histological grade for (a, d) individuals with mucinous tumours, (b, e) individuals with tumours with a mucinous component, (c, f) individuals with non-mucinous tumours. dMMR DNA mismatch repair deficient, pMMR DNA mismatch repair proficient; *p < 0.050.
Prognostic interactions of clinico-molecular variables with tumour mucinous component
Given the evidence for differential prognostic impact of tumour grade by mucinous component, we examined whether such an interaction might also exist for other clinico-molecular variables.
In univariate analyses for age at diagnosis, gender, tumour stage, ___location and BRAF mutation, a significant interaction was evident for BRAF mutation when comparing mucinous vs. non-mucinous and mucinous component vs. non-mucinous tumours (pinteraction ≤ 0.017 for both comparisons). The 5-year DFS rates among mucinous and mucinous component tumours were 83.8% and 80.9% for BRAF mutated and 75.9% and 73.9% for BRAF wild-type tumours, respectively (Fig. 2a, b), while among non-mucinous tumours DFS rates were 62.3% for BRAF mutated and 74.2% for BRAF wild-type tumours (Fig. 2c). Accordingly, BRAF mutation was not associated with outcome in multivariate analyses for mucinous and mucinous component tumours (mucinous: HR 0.82, 95% CI 0.37–1.83; p = 0.632; mucinous component: HR 0.95, 95% CI 0.44–2.05; p = 0.889; Table 3A–B), but was an independent predictor of poor prognosis in non-mucinous tumours (HR 1.93, 95% CI 1.30–2.88; p = 0.001; Table 3D). In multivariate analysis adjusted for clinico-molecular variables comparing mucinous/mucinous component vs. non-mucinous tumours, the prognostic interaction with BRAF mutation remained significant (pinteraction < 0.013).
Considering BRAF prognostic value by tumour MMR status, BRAF mutation was associated with the anticipated inferior outcomes for pMMR but not dMMR cases among non-mucinous tumours in multivariate analysis (pMMR: HR 2.61, 95% CI 1.69–4.03; p < 0.001; dMMR: HR 1.03, 95% CI 0.48–2.21; p = 0.945; Supplementary Figure S3C–D). In contrast, among mucinous/mucinous component tumours BRAF mutation was not associated with prognosis irrespective of tumour MMR status (pMMR: HR 1.26, 95% CI 0.65–2.45; p = 0.489; dMMR: HR 0.70, 95% CI 0.32–1.54; p = 0.372; Supplementary Figure S3A–B;).
No significant interactions for clinico-molecular variables were observed when comparing mucinous component and mucinous tumours (pinteraction > 0.083 for all comparisons).
Prognostic value of TIL status
Emerging findings indicate that consideration of TIL density provides a refined prognostic stratification for patients with stage II/III CRC irrespective of MMR status [36, 39,40,41].
In our cohort, TIL status data were available for 1636 patients as described previously [36], with 31.9% (522 of 1636) of cancers classified as TIL-high (≥2 TILs/ hpf). Compared with non-mucinous tumours, both mucinous component and mucinous tumours were significantly associated with TIL-high (non-mucinous: 25.3% (283/1119), mucinous component: 51.5% (102/198), mucinous: 42.9% (137/319), p < 0.001 for both comparisons); this was not due to the higher rate of dMMR in mucinous/mucinous component tumours, with the association maintained when restricting the analysis to pMMR tumours (p ≤ 0.006 for both comparisons).
Among both mucinous/mucinous component and non-mucinous tumours, TIL status stratified DFS, with TIL-high tumours exhibiting significantly better outcomes as compared with TIL-low tumours (univariate p = 0.001 for both comparisons; Fig. 3a, d). Five-year DFS rates among mucinous/mucinous component and non-mucinous, tumours were 87.9% (95% CI 83.5–92.5%) and 86.2% (95% CI 81.9–90.7%) for TIL-high tumours, and 70.8% (95% CI 64.4–76.3%) and 67.7% (95% CI 64.3–71.3%) for TIL-low tumours, respectively. The prognostic value of TIL status remained when considering mucinous and mucinous component tumours separately (Supplementary Figure S4). Moreover, in multivariate analyses TIL-high remained a strong independent predictor of good prognosis in both groups (mucinous/mucinous component: HR 0.40, 95% CI 0.23–0.67; p < 0.001; non-mucinous: HR 0.37, 95% CI 0.24–0.56; p < 0.001).
Patients are grouped into (a–c) individuals with mucinous/mucinous component tumours, (d–f) individuals with non-mucinous tumours for (a, d) the entire cohort, (b, e) dMMR tumour, and (c, f) pMMR tumours. TIL, tumour infiltrating lymphocyte; dMMR DNA mismatch repair deficient, pMMR DNA mismatch repair proficient; *p < 0.050.
Considering dMMR tumours, TIL-high status maintained its association with good prognosis among mucinous/mucinous component and non-mucinous tumours (univariate p = 0.001 for both comparisons; Fig. 3b, e). Similar prognostic trends were observed for pMMR tumours, with TIL status exhibiting borderline statistical significance for mucinous/mucinous component tumours (univariate p = 0.060; Fig. 3c) and maintaining significance for non-mucinous tumours (univariate p < 0.001; Fig. 3f). Five year DFS rates for mucinous/mucinous component CRCs were 90.5% (95% CI 85.7–95.6%) for TIL-high/dMMR tumours, 81.1% (95% CI 72.0–91.4%) for TIL-high/pMMR tumours, 73.1% (95% CI 62.9–84.9%) for TIL-low/dMMR tumours and 68.0% (95% CI 61.1–75.7%) for TIL-low/pMMR tumours, respectively. The univariate results for dMMR and pMMR tumours were reflected in multivariable analysis adjusting for clinico-molecular variables and treatment (Supplementary Table S4). Similarly, TIL-high status maintained its association with good prognosis among mucinous/mucinous component and non-mucinous tumours when stratified by grade (Supplementary Figure S5) or BRAF mutation status (Supplementary Figure S6).
In a direct comparison of the prognostic value of TIL status among mucinous/mucinous component tumours against histological grade, MMR and BRAF mutation status in multivariate Cox regression models using the Akaike Information Criterion (AIC) the TIL model was favoured in all cases, with AIC differences of 12.3 or greater indicating that there was essentially no support for grade, MMR and BRAF models being as good as the TIL model (Table 4) [43]. Corresponding results were found when using the Bayesian Information Criterion (data not shown). Accordingly, addition of TIL status significantly improved DFS models with tumour grade, MMR and BRAF variables (p < 0.05 for all comparisons, likelihood-ratio test), whereas addition of tumour grade, MMR and BRAF variables to models with TIL status did not significantly improve model fit (Table 5).
Discussion
Surveying a community-based cohort of 1643 patients with sporadic CRC, we found that tumours with a mucinous component exhibited similar clinico-molecular characteristics as mucinous tumours. Overall, tumour classification by mucinous differentiation did not stratify DFS in our cohort. Tumour MMR status, but not histological grade, stratified survival for mucinous tumours in univariate analysis consistent with WHO 2010 guidelines [1], and similar results were obtained for tumours with a mucinous component. However, neither tumour grade nor MMR status were independent prognostic variables for mucinous/mucinous component tumours in multivariate analyses. Our data indicate that the prognostic value of BRAF mutation depends upon mucinous differentiation and MMR status, with poor prognosis limited to non-mucinous pMMR tumours. Tumour classification according to TIL status was a strong independent predictor of DFS in mucinous/mucinous component tumours, superior compared with histological grade, MMR and BRAF mutation, highlighting the potential clinical utility of this categorisation.
The prevalence of mucinous tumours of ~19% in our cohort was in the upper range for studies from Western countries [5,6,7], likely related to the relatively high proportion of proximal colon tumours (~45%) in our patients. Compared with non-mucinous CRC, mucinous tumours were associated with female gender, proximal ___location, high grade, TIL-high, dMMR and BRAF mutation. Mucinous tumour associations with proximal ___location [3, 4, 6, 8, 11, 13, 15], dMMR [8,9,10,11,12,13,14,15,16] and BRAF mutation [10, 13,14,15,16] are well-established, indicating that our cases are representative of mucinous CRCs.
The prevalence of tumours with a mucinous component is less well defined, with previous surveys reporting rates of 4–7% for Asian and 20–28% for Western cohorts [8, 11, 13, 18,19,20,21]. In our cohort, 12% of patients had tumours with a mucinous component. We found that tumours with a mucinous component exhibited similar clinico-molecular characteristics as mucinous tumours, suggesting that the presence of any degree of extracellular mucin identifies a CRC subtype that is biologically distinct from non-mucinous carcinoma. In a previous large cohort analysis, Ogino et al. also concluded that in general mucinous and mucinous component tumours appeared similar, although they noted some differences for frequencies of MSI and MGMT loss [17]. Likewise, Yoon et al. reported that patients with mucinous and mucinous component tumours had overall similar clinicopathological characteristics, which differed from those with non-mucinous tumours [11]. Overall concordant observations were made in other studies [8, 18,19,20,21]. For our patient cohort, the only differentiating characteristic between mucinous and mucinous component CRCs was tumour stage, with the former associated with stage II disease. Interestingly, a similar association was evident in the cohort investigated by Langner et al. [8].
The prognostic value of tumour mucinous differentiation is controversial [2, 4,5,6,7, 11,12,13, 19, 23, 24]. A meta-analysis of 34 studies indicates only a slightly worse survival rate among mucinous CRC as compared with other cases (HR 1.06, 95% CI 1.03–1.09) [23]. Similarly, most previous studies examining tumours with a mucinous component found no evidence for a clinically relevant prognostic difference as compared with mucinous or non-mucinous tumours [8, 11, 13, 20, 22]. In agreement with these studies we observed similar outcomes by mucinous differentiation in our patients.
Our data provide some evidence to support the WHO 2010 premise that tumour MMR status, rather than histological grade, is a prognostic factor in mucinous tumours. Consistent with these guidelines, we found that MMR status stratified mucinous cancers for DFS with better outcomes in dMMR tumours in univariate analysis, while classic histological tumour grade did not stratify survival. In line with our data for MMR status, several previous studies have also reported a survival benefit for dMMR in mucinous tumours [11, 13, 14, 30, 45], although others found no survival difference [19, 46]. As observed for clinico-molecular characteristics, tumours with a mucinous component showed similar prognostic relationships as mucinous tumours, with 5-year DFS rates of 84.2% for dMMR and 69.2% for pMMR tumours, but similar outcomes by histological grade. In a comparison of mucinous and mucinous component tumours against non-mucinous tumours, the interaction between tumour grade and mucinous differentiation was significant. Nonetheless, in multivariate analysis combining mucinous and mucinous component tumours, dMMR status did not reach statistical significance as an independent prognostic predictor. This latter finding is consistent with the updated recommendation in the WHO 2019 guidelines [32], but our data do not support the contention that conventional histological grading should be applied to mucinous tumours.
About 10% of CRCs harbour BRAF (V600E) mutations [47], and a recent meta-analysis of seven phase III clinical trials reported poorer survival for BRAF mutated stage II/III tumours compared with BRAF wild-type tumours [48]. Moreover, there is evidence that BRAF mutated pMMR tumours constitute a particularly poor prognostic group [27,28,29]. Our findings indicate that BRAF mutation prognostic value in stage II/III CRCs depends on mucinous differentiation and MMR status, with poor prognosis limited to non-mucinous pMMR tumours. Consistent with our findings, Andrici et al. previously reported similar 5-year survival rates for BRAF wild-type and mutated pMMR mucinous CRCs [14].
There are strong data to support host immune response against tumour as a prognostic indicator in CRC patients [36, 39,40,41]. Our study indicates that the prognostic value of TIL classification is retained in stage II/III tumours with mucinous differentiation, with TIL-high a strong independent predictor of good prognosis. In our cohort, the most favourable outcomes were observed for TIL-high dMMR tumours, whilst TIL-low tumours had poor outcomes irrespective of MMR status. Notably, TIL status was a superior predictor of outcome compared with histological grade, MMR and BRAF mutation. Addition of histological grade, MMR and BRAF mutation did not improve models with TIL status. These findings highlight that a standardised method of assessment of TILs should be considered for inclusion in routine reporting of CRCs irrespective of tumour mucinous component. We have previously shown that the TIL method applied in this study has good intraobserver and interobserver reproducibility (κ statistic 0.772 and 0.666, respectively) [36]. Immunohistochemical detection of TILs or the application of digital image analysis may improve scoring reproducibility, although a revised cut-point would need to be determined for these methods.
Strengths of our study include a large cohort size with detailed treatment and long-term follow-up data, focusing on stage II/III patients. Recruitment of patients occurred at multiple hospital sites capturing a broad population. DFS is considered an appropriate sensitive endpoint for early-stage CRC, and in contrast to overall survival, is less influenced by disparities in comorbidities, treatments for recurrent disease or deaths from causes other than cancer. Tumour mucinous component was scored by a single study pathologist, with good interobserver agreement on re-examination of a subset of cases by two additional pathologists. Our large cohort size allowed for detailed comparisons of clinico-molecular features and survival between the prevalent non-mucinous and less common mucinous and mucinous component subtypes. With integrated consideration of tumour histopathologic and molecular features entering clinical care, empirical data to support clinical cut-offs for tumour classification and analyses to pinpoint confounders and prognostic interactions between variables are becoming increasing important. Our study examined these pertinent issues for tumour mucinous component, challenging the utility of the current proportion-based definition of mucinous tumours and highlighting the differential prognostic values for tumour grade and BRAF mutation. To our knowledge, none of the previous studies on global TIL status in CRC has specifically assessed tumours with mucinous differentiation.
Several limitations of our study deserve comment. First, because our patient cohort was not derived from a clinical trial, chemotherapy treatment and follow-up were performed as per routine care without mandated standardisation. Second, given the low prevalence of mucinous and mucinous component differentiation, no independent large cohort was available to further validate our findings, although many of our observations were consistent with existing literature. Third our analysis of host immune response to tumour did not allow us to distinguish types of T-cell populations or consider innate immune infiltrates which may carry additional prognostic information [37, 49]. Finally, other molecular events or epidemiological factors which were not examined in this study may impact prognosis or chemosensitivity within tumour mucinous subtypes. These may include variables such as chromosome instability [50], body mass index [51] and smoking [52].
In conclusion, our study indicates that the presence of any mucinous differentiation, irrespective of the extent, demarcates CRCs with specific clinico-molecular features indicating that these tumours are distinct from non-mucinous adenocarcinomas. Mucinous tumours and tumours with a mucinous component showed marked similarities for prognostic interactions, with evidence that neither tumour grade nor BRAF mutation provide prognostic information in these tumours. Our results support the consideration of a standardised method to evaluate TIL status in patients with stage II/III CRC to guide patient management, irrespective of the degree of mucinous differentiation. Although further studies are required to confirm our findings, our data provide useful information on mucinous differentiation in clinical and pathological practice of CRC.
References
Bosman FT, World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer; 2010.
Kanemitsu Y, Kato T, Hirai T, Yasui K, Morimoto T, Shimizu Y, et al. Survival after curative resection for mucinous adenocarcinoma of the colorectum. Dis Colon Rectum. 2003;46:160–7.
Yamaguchi T, Taniguchi H, Fujita S, Sekine S, Yamamoto S, Akasu T, et al. Clinicopathological characteristics and prognostic factors of advanced colorectal mucinous adenocarcinoma. Histopathology. 2012;61:162–9.
Park JS, Huh JW, Park YA, Cho YB, Yun SH, Kim HC, et al. Prognostic comparison between mucinous and nonmucinous adenocarcinoma in colorectal cancer. Medicine. 2015;94:e658.
Xie L, Villeneuve PJ, Shaw A. Survival of patients diagnosed with either colorectal mucinous or non-mucinous adenocarcinoma: a population-based study in Canada. Int J Oncol. 2009;34:1109–15.
Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol. 2012;19:2814–21.
Catalano V, Loupakis F, Graziano F, Bisonni R, Torresi U, Vincenzi B, et al. Prognosis of mucinous histology for patients with radically resected stage II and III colon cancer. Ann Oncol. 2012;23:135–41.
Langner C, Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Schlemmer A, et al. Mucinous differentiation in colorectal cancer-indicator of poor prognosis? Histopathology. 2012;60:1060–72.
Greenson JK, Bonner JD, Ben-Yzhak O, Cohen HI, Miselevich I, Resnick MB, et al. Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol. 2003;27:563–70.
Tanaka H, Deng G, Matsuzaki K, Kakar S, Kim GE, Miura S, et al. BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more frequently and concordantly in mucinous than non-mucinous colorectal cancer. Int J Cancer. 2006;118:2765–71.
Yoon YS, Kim J, Hong SM, Lee JL, Kim CW, Park IJ, et al. Clinical implications of mucinous components correlated with microsatellite instability in patients with colorectal cancer. Colorectal Dis. 2015;17:O161–7.
Wang MJ, Ping J, Li Y, Holmqvist A, Adell G, Arbman G, et al. Prognostic significance and molecular features of colorectal mucinous adenocarcinomas: a strobe-compliant study. Medicine. 2015;94:e2350.
Inamura K, Yamauchi M, Nishihara R, Kim SA, Mima K, Sukawa Y, et al. Prognostic significance and molecular features of signet-ring cell and mucinous components in colorectal carcinoma. Ann Surg Oncol. 2015;22:1226–35.
Andrici J, Farzin M, Sioson L, Clarkson A, Watson N, Toon CW, et al. Mismatch repair deficiency as a prognostic factor in mucinous colorectal cancer. Mod Pathol. 2016;29:266–74.
Liddell C, Droy-Dupre L, Metairie S, Airaud F, Volteau C, Bezieau S, et al. Mapping clinicopathological entities within colorectal mucinous adenocarcinomas: a hierarchical clustering approach. Mod Pathol. 2017;30:1177–89.
Reynolds IS, Furney SJ, Kay EW, McNamara DA, Prehn JHM, Burke JP. Meta-analysis of the molecular associations of mucinous colorectal cancer. Br J Surg. 2019;106:682–91.
Ogino S, Brahmandam M, Cantor M, Namgyal C, Kawasaki T, Kirkner G, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol. 2006;19:59–68.
Tozawa E, Ajioka Y, Watanabe H, Nishikura K, Mukai G, Suda T, et al. Mucin expression, p53 overexpression, and peritumoral lymphocytic infiltration of advanced colorectal carcinoma with mucus component: is mucinous carcinoma a distinct histological entity? Pathol Res Pr. 2007;203:567–74.
Kim SH, Shin SJ, Lee KY, Kim H, Kim TI, Kang DR, et al. Prognostic value of mucinous histology depends on microsatellite instability status in patients with stage III colon cancer treated with adjuvant FOLFOX chemotherapy: a retrospective cohort study. Ann Surg Oncol. 2013;20:3407–13.
Lee DW, Han SW, Lee HJ, Rhee YY, Bae JM, Cho NY, et al. Prognostic implication of mucinous histology in colorectal cancer patients treated with adjuvant FOLFOX chemotherapy. Br J Cancer. 2013;108:1978–84.
Ooki A, Akagi K, Yatsuoka T, Asayama M, Hara H, Yamamoto G, et al. Inverse effect of mucinous component on survival in stage III colorectal cancer. J Surg Oncol. 2014;110:851–7.
Gonzalez RS, Cates JMM, Washington K. Associations among histological characteristics and patient outcomes in colorectal carcinoma with a mucinous component. Histopathology. 2019;74:406–14.
Verhulst J, Ferdinande L, Demetter P, Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol. 2012;65:381–8.
Tarantino I, Huttner FJ, Warschkow R, Schmied BM, Diener MK, Ulrich A. Prognostic relevance of mucinous subtype in a population-based propensity score analysis of 40,083 rectal cancer patients. Ann Surg Oncol. 2016;23:1576–86.
Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N. Engl J Med. 2000;342:69–77.
Benatti P, Gafa R, Barana D, Marino M, Scarselli A, Pedroni M, et al. Microsatellite instability and colorectal cancer prognosis. Clin Cancer Res. 2005;11:8332–40.
Lochhead P, Kuchiba A, Imamura Y, Liao X, Yamauchi M, Nishihara R, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151–6.
Toon CW, Chou A, DeSilva K, Chan J, Patterson J, Clarkson A, et al. BRAFV600E immunohistochemistry in conjunction with mismatch repair status predicts survival in patients with colorectal cancer. Mod Pathol. 2014;27:644–50.
Seppala TT, Bohm JP, Friman M, Lahtinen L, Vayrynen VM, Liipo TK, et al. Combination of microsatellite instability and BRAF mutation status for subtyping colorectal cancer. Br J Cancer. 2015;112:1966–75.
Leopoldo S, Lorena B, Cinzia A, Gabriella DC, Angela Luciana B, Renato C, et al. Two subtypes of mucinous adenocarcinoma of the colorectum: clinicopathological and genetic features. Ann Surg Oncol. 2008;15:1429–39.
Jung SH, Kim SH, Kim JH. Prognostic impact of microsatellite instability in colorectal cancer presenting with mucinous, signet-ring, and poorly differentiated cells. Ann Coloproctol. 2016;32:58–65.
WHO Classification of Tumours Editorial Board, World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of the digestive system. 5th ed. Lyon: International Agency for Research on Cancer; 2019.
Klintrup K, Makinen JM, Kauppila S, Vare PO, Melkko J, Tuominen H, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645–54.
Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–20.
Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang G, et al. Tumour-infiltrating inflammation and prognosis in colorectal cancer: systematic review and meta-analysis. Br J Cancer. 2014;110:1595–605.
Williams DS, Mouradov D, Jorissen RN, Newman MR, Amini E, Nickless DK, et al. Lymphocytic response to tumour and deficient DNA mismatch repair identify subtypes of stage II/III colorectal cancer associated with patient outcomes. Gut. 2019;68:465–74.
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and ___location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4.
Mlecnik B, Bindea G, Kirilovsky A, Angell HK, Obenauf AC, Tosolini M, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8:327ra26.
Wirta EV, Seppala T, Friman M, Vayrynen J, Ahtiainen M, Kautiainen H, et al. Immunoscore in mismatch repair-proficient and -deficient colon cancer. J Pathol Clin Res. 2017;3:203–13.
Rozek LS, Schmit SL, Greenson JK, Tomsho LP, Rennert HS, Rennert G, et al. Tumor-Infiltrating lymphocytes, crohn’s-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016;108: https://www.ncbi.nlm.nih.gov/pubmed/27172903.
Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, et al. Integrative analyses of colorectal cancer show immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity. 2016;44:698–711.
Day F, Muranyi A, Singh S, Shanmugam K, Williams D, Byrne D, et al. A mutant BRAF V600E-specific immunohistochemical assay: correlation with molecular mutation status and clinical outcome in colorectal cancer. Target Oncol. 2015;10:99–109.
Burnham KP, Anderson DR, Burnham KP. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. New York: Springer; 2002.
Gill S, Sargent D. End points for adjuvant therapy trials: has the time come to accept disease-free survival as a surrogate end point for overall survival? Oncologist. 2006;11:624–9.
Kakar S, Aksoy S, Burgart LJ, Smyrk TC. Mucinous carcinoma of the colon: correlation of loss of mismatch repair enzymes with clinicopathologic features and survival. Mod Pathol. 2004;17:696–700.
Kazama Y, Watanabe T, Kanazawa T, Kazama S, Tada T, Tanaka J, et al. Mucinous colorectal cancers with chromosomal instability: a biologically distinct and aggressive subtype. Diagn Mol Pathol. 2006;15:30–4.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Zhu L, Dong C, Cao Y, Fang X, Zhong C, Li D, et al. Prognostic role of BRAF mutation in stage II/III colorectal cancer receiving curative resection and adjuvant chemotherapy: a meta-analysis based on randomized clinical trials. PLoS ONE. 2016;11:e0154795.
Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228:1404–12.
Mouradov D, Domingo E, Gibbs P, Jorissen RN, Li S, Soo PY, et al. Survival in stage II/III colorectal cancer is independently predicted by chromosomal and microsatellite instability, but not by specific driver mutations. Am J Gastroenterol. 2013;108:1785–93.
Sinicrope FA, Foster NR, Yothers G, Benson A, Seitz JF, Labianca R, et al. Body mass index at diagnosis and survival among colon cancer patients enrolled in clinical trials of adjuvant chemotherapy. Cancer. 2013;119:1528–36.
Parajuli R, Bjerkaas E, Tverdal A, Le Marchand L, Weiderpass E, Gram IT. Cigarette smoking and colorectal cancer mortality among 602,242 Norwegian males and females. Clin Epidemiol. 2014;6:137–45.
Acknowledgements
The authors thank the Victorian Cancer BioBank and BioGrid Australia for provision of patient specimens and clinical data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare. This work was supported by a Cancer Australia Project Grant (APP1120882), a Cancer Council Victoria Grant-in-Aid (1060964), the Victorian Government’s Operational Infrastructure Support Program, a University of Melbourne Department of Pathology Career Development Award, and an Austin Medical Research Foundation Project Grant. O.M.S. is a NHMRC Senior Research Fellow (APP1136119).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Williams, D.S., Mouradov, D., Newman, M.R. et al. Tumour infiltrating lymphocyte status is superior to histological grade, DNA mismatch repair and BRAF mutation for prognosis of colorectal adenocarcinomas with mucinous differentiation. Mod Pathol 33, 1420–1432 (2020). https://doi.org/10.1038/s41379-020-0496-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0496-1
This article is cited by
-
Automated deep learning-based assessment of tumour-infiltrating lymphocyte density determines prognosis in colorectal cancer
Journal of Translational Medicine (2025)
-
A nomogram for predicting cancer-specific survival in patients with rectal mucinous adenocarcinoma following surgery
Scientific Reports (2025)
-
Associations of mucinous differentiation and mucin expression with immune cell infiltration and prognosis in colorectal adenocarcinoma
British Journal of Cancer (2025)
-
Identification and validation of a novel six-gene signature based on mucinous adenocarcinoma-related gene molecular typing in colorectal cancer
Discover Oncology (2024)
-
Immune checkpoint blockade therapy for BRAF mutant metastatic colorectal cancer: the efficacy, new strategies, and potential biomarkers
Discover Oncology (2023)