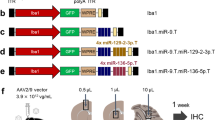
Abstract
Autism spectrum disorder (ASD) is a prevalent neurodevelopmental disorder. The microglia activation is a hallmark of ASD, which involves increased glycolysis. Elevated glycolysis regardless of oxygen availability, known as “Warburg effect”, is crucial to pathogenesis in neuropsychiatric disorders. Psychiatric risk gene MIR137 plays an important role in neurogenesis and neuronal maturation, but the impact on neuroinflammation and glucose metabolism remains obscure. Extracellular vesicles (EVs) can delivery miR-137 crossing the blood-brain barrier. Meanwhile, EVs can help miR-137 avoid being rapidly degraded by endogenous nucleases. Here, after first detecting miR-137 decreased both in the peripheral blood of individuals with ASD and the serum and cerebellum of BTBR mice, we demonstrated that microglia activation, the level of lactate and key enzymes (HK2, PKM2 and LDHA) involved in glycolysis were increased significantly in BTBR mice. Of particular note, EVs engineered by rabies virus glycoprotein (RVG) could promote the miR-137 (RVG-miR137-EVs) targeted to the brain accurately, and alleviated autism-like behaviors. Pro-inflammatory activation of BTBR mice was considerably inhibited by RVG-miR137-EVs via tail vein administration, accompanied by decreased lactate production. Mechanically, these effects were attributed to TLR4, the key target gene, which was regulated by miR-137. The TLR4/NF-κB pathway was inhibited, subsequently reducing HIF-1α and repressing the transcription of HK2, PKM2 and LDHA involved in glycolysis. Pharmacological inhibition of glycolysis and TLR4 attenuated microglial activation and lactate production, ultimately improved autism-like behaviors of BTBR mice. In conclusion, our results indicated that miR-137 could alleviate autism-like behaviors by HIF-1α-mediated adaptive metabolic changes in glycolysis and neuroinflammation.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Further information and request for resources and reagent should be directed to corresponding author on reasonable request.
References
Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis Primers. 2020;6:5.
Edmonson C, Ziats MN, Rennert OM. Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol Autism. 2014;5:3.
Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, et al. Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry. 2010;68:368–76.
Reed MD, Yim YS, Wimmer RD, Kim H, Ryu C, Welch GM, et al. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577:249–53.
Suzuki K, Sugihara G, Ouchi Y, Nakamura K, Futatsubashi M, Takebayashi K, et al. Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry. 2013;70:49–58.
Kaushik DK, Bhattacharya A, Mirzaei R, Rawji KS, Ahn Y, Rho JM, et al. Enhanced glycolytic metabolism supports transmigration of brain-infiltrating macrophages in multiple sclerosis. J Clin Invest. 2019;129:3277–92.
Li Y, Lu B, Sheng L, Zhu Z, Sun H, Zhou Y, et al. Hexokinase 2-dependent hyperglycolysis driving microglial activation contributes to ischemic brain injury. J Neurochem. 2018;144:186–200.
Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280.
Traxler L, Herdy JR, Stefanoni D, Eichhorner S, Pelucchi S, Szucs A, et al. Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease. Cell Metab. 2022;34:1248–1263.e1246.
Huang ZP, Liu SF, Zhuang JL, Li LY, Li MM, Huang YL, et al. Role of microglial metabolic reprogramming in Parkinson’s disease. Biochem Pharmacol. 2023;213:115619.
Weissman JR, Kelley RI, Bauman ML, Cohen BH, Murray KF, Mitchell RL, et al. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. 2008;3:e3815.
El Fotoh W, El Naby SAA, Abd El Hady NMS. Autism spectrum disorders: the association with inherited metabolic disorders and some trace elements. a retrospective study. CNS Neurol Disord Drug Targets. 2019;18:413–20.
Khemakhem AM, Frye RE, El-Ansary A, Al-Ayadhi L, Bacha AB. Novel biomarkers of metabolic dysfunction is autism spectrum disorder: potential for biological diagnostic markers. Metab Brain Dis. 2017;32:1983–97.
Correia C, Coutinho AM, Diogo L, Grazina M, Marques C, Miguel T, et al. Brief report: High frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord. 2006;36:1137–40.
Hagihara H, Catts VS, Katayama Y, Shoji H, Takagi T, Huang FL, et al. Decreased brain pH as a shared endophenotype of psychiatric disorders. Neuropsychopharmacology. 2018;43:459–68.
Liu S, Li A, Liu Y, Li J, Wang M, Sun Y, et al. MIR137 polygenic risk is associated with schizophrenia and affects functional connectivity of the dorsolateral prefrontal cortex. Psychol Med. 2020;50:1510–8.
Strazisar M, Cammaerts S, van der Ven K, Forero DA, Lenaerts AS, Nordin A, et al. MIR137 variants identified in psychiatric patients affect synaptogenesis and neuronal transmission gene sets. Mol Psychiatry. 2015;20:472–81.
Duan J, Shi J, Fiorentino A, Leites C, Chen X, Moy W, et al. A rare functional noncoding variant at the GWAS-implicated MIR137/MIR2682 locus might confer risk to schizophrenia and bipolar disorder. Am J Hum Genet. 2014;95:744–53.
Pinto D, Delaby E, Merico D, Barbosa M, Merikangas A, Klei L, et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94:677–94.
Cheng Y, Wang ZM, Tan W, Wang X, Li Y, Bai B, et al. Partial loss of psychiatric risk gene Mir137 in mice causes repetitive behavior and impairs sociability and learning via increased Pde10a. Nat Neurosci. 2018;21:1689–703.
van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213–28.
Kaushik S, Cuervo AM. Proteostasis and aging. Nat Med. 2015;21:1406–15.
Momen-Heravi F, Bala S, Bukong T, Szabo G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine: Nanotechnology, Biology, and Medicine. 2014;10:1517–27.
Wiklander OP, Nordin JZ, O’Loughlin A, Gustafsson Y, Corso G, Mäger I, et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5.
Karimi N, Cvjetkovic A, Jang SC, Crescitelli R, Hosseinpour Feizi MA, Nieuwland R, et al. Detailed analysis of the plasma extracellular vesicle proteome after separation from lipoproteins. Cell Mol Life Sci. 2018;75:2873–86.
Shpyleva S, Ivanovsky S, de Conti A, Melnyk S, Tryndyak V, Beland FA, et al. Cerebellar oxidative DNA damage and altered DNA methylation in the BTBR T+tf/J mouse model of autism and similarities with human post mortem cerebellum. PLoS ONE. 2014;9:e113712.
Ginsberg MR, Rubin RA, Natowicz MR. Patterning of regional gene expression in autism: new complexity. Sci Rep. 2013;3:1831.
Irimia M, Weatheritt RJ, Ellis JD, Parikshak NN, Gonatopoulos-Pournatzis T, Babor M, et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell. 2014;159:1511–23.
Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, et al. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–4.
Nakata M, Kimura R, Funabiki Y, Awaya T, Murai T, Hagiwara M. MicroRNA profiling in adults with high-functioning autism spectrum disorder. Mol Brain. 2019;12:82.
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47.
Ito K, Murphy D. Application of ggplot2 to Pharmacometric Graphics. CPT Pharmacomet Syst Pharmacol. 2013;2:e79.
Gene Ontology C. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43:D1049–1056.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30.
Sherman BT, Hao M, Qiu J, Jiao X, Baseler MW, Lane HC, et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022;50:W216–w221.
Psych EC, Akbarian S, Liu C, Knowles JA, Vaccarino FM, Farnham PJ, et al. The PsychENCODE project. Nat Neurosci. 2015;18:1707–12.
Qin Q, Fan L, Zeng X, Zheng D, Wang H, Li M, et al. Mesenchymal stem cell-derived extracellular vesicles alleviate autism by regulating microglial glucose metabolism reprogramming and neuroinflammation through PD-1/PD-L1 interaction. J Nanobiotechnol. 2025;23:201.
Zeng X, Fan L, Qin Q, Zheng D, Wang H, Li M, et al. Exogenous PD-L1 binds to PD-1 to alleviate and prevent autism-like behaviors in maternal immune activation-induced male offspring mice. Brain, Behavior, Immun. 2024;122:527–46.
Fan L, Zeng X, Jiang Y, Zheng D, Wang H, Qin Q, et al. Yigansan ameliorates maternal immune activation-induced autism-like behaviours by regulating the IL-17A/TRAF6/MMP9 pathway: Network analysis and experimental validation. Phytomedicine. 2024;128:155386.
Cristiano C, Pirozzi C, Coretti L, Cavaliere G, Lama A, Russo R, et al. Palmitoylethanolamide counteracts autistic-like behaviours in BTBR T+tf/J mice: contribution of central and peripheral mechanisms. Brain, Behavior, Immun. 2018;74:166–75.
Fodelianaki G, Lansing F, Bhattarai P, Troullinaki M, Zeballos MA, Charalampopoulos I, et al. Nerve Growth Factor modulates LPS - induced microglial glycolysis and inflammatory responses. Exp Cell Res. 2019;377:10–16.
Jia X, Gao Z, Hu H. Microglia in depression: current perspectives. Sci China Life Sci. 2021;64:911–25.
Van Overwalle F, Manto M, Cattaneo Z, Clausi S, Ferrari C, Gabrieli JDE, et al. Consensus paper: cerebellum and social cognition. Cerebellum. 2020;19:833–68.
Stoodley CJ. The cerebellum and neurodevelopmental disorders. Cerebellum. 2016;15:34–37.
Hu Y, Mai W, Chen L, Cao K, Zhang B, Zhang Z, et al. mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia. 2020;68:1031–45.
Pan RY, Ma J, Kong XX, Wang XF, Li SS, Qi XL, et al. Sodium rutin ameliorates Alzheimer’s disease-like pathology by enhancing microglial amyloid-beta clearance. Sci Adv. 2019;5:eaau6328.
Baik SH, Kang S, Lee W, Choi H, Chung S, Kim JI, et al. A breakdown in metabolic reprogramming causes microglia dysfunction in Alzheimer’s disease. Cell Metab. 2019;30:493–507.e496.
Qiao H, He X, Zhang Q, Yuan H, Wang D, Li L, et al. Alpha-synuclein induces microglial migration via PKM2-dependent glycolysis. Int J Biol Macromol. 2019;129:601–7.
Vallee A, Vallee JN. Warburg effect hypothesis in autism Spectrum disorders. Mol Brain. 2018;11:1.
Heidari A, Yazdanpanah N, Rezaei N. The role of Toll-like receptors and neuroinflammation in Parkinson’s disease. J Neuroinflammation. 2022;19:135.
Ni H, Liu M, Cao M, Zhang L, Zhao Y, Yi L, et al. Sinomenine regulates the cholinergic anti-inflammatory pathway to inhibit TLR4/NF-kappaB pathway and protect the homeostasis in brain and gut in scopolamine-induced Alzheimer’s disease mice. Biomed Pharmacother. 2024;171:116190.
Liu M, Xu Z, Wang L, Zhang L, Liu Y, Cao J, et al. Cottonseed oil alleviates ischemic stroke injury by inhibiting the inflammatory activation of microglia and astrocyte. J Neuroinflammation. 2020;17:270.
Murphy CE, Walker AK, O’Donnell M, Galletly C, Lloyd AR, Liu D, et al. Peripheral NF-kappaB dysregulation in people with schizophrenia drives inflammation: putative anti-inflammatory functions of NF-kappaB kinases. Transl Psychiatry. 2022;12:21.
Li D, Wang Y, Jin X, Hu D, Xia C, Xu H, et al. NK cell-derived exosomes carry miR-207 and alleviate depression-like symptoms in mice. J Neuroinflammation. 2020;17:126.
Pan T, Sun S, Chen Y, Tian R, Chen E, Tan R, et al. Immune effects of PI3K/Akt/HIF-1α-regulated glycolysis in polymorphonuclear neutrophils during sepsis. Crit Care. 2022;26:29.
Lu H, Lin J, Xu C, Sun M, Zuo K, Zhang X, et al. Cyclosporine modulates neutrophil functions via the SIRT6-HIF-1α-glycolysis axis to alleviate severe ulcerative colitis. Clin Transl Med. 2021;11:e334.
Acknowledgements
We sincerely thank the assistance provided from Province Key Laboratory of Children development and genetic research (Harbin Medical University, Heilongjiang, China) and Key Laboratory of Preservation of Human Genetic Resources and Disease Control in China, Ministry of Education (Harbin Medical University, Heilongjiang, China).
Funding
This study was supported by grants from the National Natural Science Foundation of China (81973068); the Open Project Program of Key Laboratory of Preservation of Human Genetic Resources and Disease Control in China (LPHGRDC2021-004); Postgraduate Research & Practice Innovation Program of Harbin Medical University (YJSCX2023-19HYD); the Excellent Young Teachers’ Basic Research Support Program of Heilongjiang Province (YQJH2023040); Heilongjiang Province Postdoctoral Start-up Fund (LBH-Q19029).
Author information
Authors and Affiliations
Contributions
QQ, SL and LJW conceptualized and designed experiments for this research. QQ performed molecular experiments; analyzed the data; and wrote and edited the original manuscript. QQ and LLF preprocessed and analyzed the molecular experiments. MYL, LLF and XZ performed bioinformatic analysis. LX and XRM performed cell culture experiments. DYZ, HW and YTJ performed mice breeding and mice behavior experiments. SL obtained funding. SL and LJW critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal studies and experimental procedures were approved by the Ethics Committee of Harbin Medical University (HMUIRB20210002) and were performed according to the guidelines of the Institutional Animal Care and Use Committee of the university. Additionally, the number of mice used in the experiments was minimized as much as possible in accordance with the 3R-principle (replacement, reduction, and refinement). We carried out euthanasia according to regulations for all mice that reached the end of their lives.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qin, Q., Li, M., Fan, L. et al. RVG engineered extracellular vesicles-transmitted miR-137 improves autism by modulating glucose metabolism and neuroinflammation. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-02988-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-02988-0