Abstract
Internet Gaming Disorder (IGD) is marked by impaired psychological and social functioning but remains without effective treatments. Cue exposure therapy (CET) is typically administered during wakefulness to help extinguish addictive memories. However, recent studies suggest that sleep may be an optimal state for memory modulation. This study aimed to assess the efficacy of repeated closed-loop exposure to game sounds during UP-state of slow-wave sleep (SWS) on IGD. 84 participants meeting DSM-5 criteria for IGD were randomly assigned to sleep intervention/control groups (SIG/SCG) or awake intervention/control groups (AIG/ACG) with two consecutive days of intervention. During SWS of two intervention nights, around 300 sounds were exposed at slow-wave UP-state. While the awake groups received similar auditory cue exposure during the awake state for two consecutive days. Cravings, playtime, and P300 amplitude in the cue reactivity task were recorded at baseline, post-intervention, and follow-up intervals (1, 2, 3, weeks, and 1 month). Results showed that the SIG significantly reduced cravings (p < 0.001), and playtime (p = 0.009) at post-intervention and follow-up, whereas awake CET showed no effect. The SIG exhibited higher low-frequency and early spindle power, along with lower late spindle power after sound exposure. Notably, the linear increase in sound-elicited late spindle power across the 20 intervention blocks over two experiment nights was positively correlated with reduced cravings post-intervention (r = 0.54, p = 0.015), especially among participants achieving a craving reduction greater than 30% after one month. Our findings suggest that closed-loop auditory exposure during SWS presents a promising, non-invasive intervention strategy for treating IGD, potentially exerting its effects by modulating late spindle power.
Similar content being viewed by others
Introduction
Internet Gaming Disorder (IGD) is characterized by impaired control over gaming, an increasing priority given to gaming, and the continuation or escalation of gaming despite negative consequences [1, 2]. IGD affects approximately 8.8% of adolescents and 10.4% of young adults worldwide [3]. It is included in both the 11th Revision of the International Classification of Diseases (ICD-11) and the 5th edition of The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [2]. However, effective treatments for IGD remain limited.
The persistent addictive memory is thought to be the core factor driving psychological cravings and relapse, even after prolonged abstinence [4, 5]. Addictive disorders have been treated by facilitating the extinction of addiction-related memories through repeated exposure to the addictive substance or related cues, a method known as cue exposure therapy (CET) [6]. CET has been widely used to treat addictions such as smoking and alcohol [7,8,9,10,11,12,13,14], with positive effects on reducing cravings [7, 8, 10,11,12]. However, recent meta-analyses have suggested that CET has limited efficacy in reducing cravings and preventing relapse for substance addictions [15, 16]. Evidence regarding the use of CET to address addictive memories in IGD is scarce. Only one recent study showed that a CET-based approach, specifically a retrieval-extinction procedure, could successfully reduce cravings in individuals with IGD [17]. Therefore, further research on enhanced CET-based therapies for IGD is needed.
Sleep is considered an unconscious state for the brain, free from external distractors and tasks [18]. The memory-related neural connections could be reorganized (strength or extinction) during sleep [19, 20]. Recent studies suggest that sleep is an ideal time for modulating memories through the presentation of sensory cues, such as tones or odors previously associated with specific stimuli or events- known as target memory reactivation (TMR) [21,22,23,24,25]. Research has shown that repeated cue exposure during slow-wave sleep (SWS) can exert therapeutic effects on conditions such as post-traumatic stress disorder (PTSD) [26] or smoking addiction [27], and can promote fear memory extinction [28, 29]. Thus, sleep, particularly SWS, could be a promising period to eliminate and rewrite pathological memories unconsciously in IGD.
During SWS sleep, slow oscillations [SOs, < 2 Hz] and spindles (12-17 Hz) are key electroencephalogram (EEG) signatures [30,31,32]. Both SOs and spindles play critical roles in the reactivation and consolidation of memories [21, 33,34,35]. SOs are thought to act as global pacemakers of brain activity during sleep, alternating between phases of neuronal excitability (UP states) and inhibition (DOWN states) [33]. Notably, SO UP-states are associated with increased brain-wide coherence following the learning of declarative memories [33, 36, 37], which are essential for processing of information during sleep [38, 39]. Based on these findings, we hypothesize that the SO UP-state may represent an optimal period for intervening in the extinction of targeted addictive memories.
This study aims to investigate whether repeated exposure to game-related auditory cues (gaCET) during SO UP-states in SWS effectively reduces cravings and playtime in individuals with IGD. We also compare these effects of gaCET during wakefulness. Additionally, we explore the neuroelectrophysiological mechanisms underlying the effects of gaCET during sleep on IGD.
Methods
Ethics approval and consent to participate
This randomized controlled trial was conducted in accordance with the Declaration of Helsinki and was approved by the Peking University Institutional Review Board (IRB00001052-23170), with registration at the Chinese Clinical Trial Registry (ChiCTR2400089928). The study was performed in Beijing from June 1, 2023, to May 30, 2024. All participants provided written informed consent prior to enrollment and received a monetary compensation of 500 RMB after completing four experimental visits.
Participants
The sleep group consisted of 42 IGD individuals from universities in Beijing. The inclusion criteria were: (1) playing “Honor of Kings” (a massively multiplayer online competitive game widely popular among Chinese college students [40]) for more than 21 h per week, (2) meeting 5 or more of the 9 diagnostic criteria for IGD according to DSM-5 [2], as assessed by trained medical researchers, (3) being right-handed with normal hearing. Exclusion criteria included: (1) the Beck Anxiety Inventory score≥50, (2) the Patient Health Questionnaire-9 score≥20, (3) the Insomnia Severity Index score≥15, (4) had been diagnosed as chronic neurological or psychiatric disorders, and (5) other substance dependencies. Participants were randomly assigned to either the sleep intervention group (SIG) or the sleep control group (SCG) in a 1:1 ratio according to a computer-generated randomization schedule. Further details are provided in the Supplement.
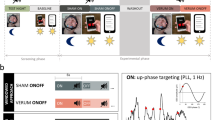
The awake group consisted of 42 IGD individuals who were randomly and evenly assigned to either the awake intervention group (AIG) or the awake control group (ACG). The inclusion and exclusion criteria were identical to those of the sleep groups. The CONSORT flow diagram and experimental design are presented in Fig. 1.
A CONSORT flow diagram. B Experimental design. a. Timeline of sleep groups: Participants were split into two groups (SIG and SCG) and spent two consecutive nights in the sleep laboratory. SIG participants were exposed to approximately 300 game-related sounds per night, while SCG participants heard around 300 non-game-related sounds. Online game craving questionnaires and cue reactivity tasks were administered before and after the intervention. b. Timeline for awake groups. Participants were divided into two groups (AIG and ACG), On day 1, they completed online game craving questionnaires, a cue reactivity task, and a 50-min cue-exposure intervention (approximately 300 game-related sounds for AIG and 300 non-game-related sounds for ACG). On day 2, the cue-exposure intervention was repeated. On day 3, participants took the cue reactivity task and completed the craving questionnaires again. All four groups then filled out the online game craving questionnaires at 1 (T2), 2 (T3), 3 weeks (T4), and at 1 month (T5) post-intervention follow-up. Participants returned to the laboratory for the cue reactivity task 1 month later. SIG, sleep intervention group; SCG, sleep control group; AIG, awake intervention group; ACG, awake control group.
Interventions and procedures
In the pre-experiment questionnaire survey, we found that game players’ cravings peaked between 9 p.m. and 11 p.m. by asking about the time they most wanted to play games. To minimize the influence of environmental and circadian factors on cravings, all participants completed online questionnaires about game cravings and playtime between 9 and 11 p.m. on the evening prior to the experiment (T0).
Sleep groups
On the first experimental night, participants arrived at the sleep laboratory around 10 p.m. and were prepared for EEG recordings. They then completed a cue reactivity task (T0). Afterward, they went to bed, with lights turned off at 11 p.m. Sounds were played when a SO UP-state was detected after the onset of SWS. The SIG was exposed to approximately 300 game sounds, while the SCG heard around 300 non-game sounds, both of which had been presented during the cue reactivity task prior to sleep. After 8.5 h of sleep, participants were gently awakened around 7:30 a.m. The protocol for the second consecutive experimental night followed the same procedure as the first night. On the morning following the two experimental nights (T1), participants completed the cue reactivity task while undergoing EEG recording (15-min after awakening). They then filled out online questionnaires about cravings between 9 and 11 p.m. (T1).
Awake groups
On the first day, participants arrived at the laboratory to complete the cue reactivity task (T0). They then underwent approximately 50 min of gaCET at almost the same time each day for two consecutive days. For the AIG, this intervention involved listening to 300 game-related sounds, while the ACG listened to 300 non-game sounds. On the morning following the two experimental days (T1), participants returned to the lab to complete the cue reactivity task under EEG recording. They then filled out online questionnaires between 9 and 11 p.m. (T1).
Follow-up tests
All participants then completed online questionnaires on game craving and playtime between 9 and 11 p.m. at 1 week (T2), 2 weeks (T3), 3 weeks (T4), and 1 month (T5) post-intervention. At the 1-month follow-up (T5), participants returned to the laboratory to complete the cue reactivity task under EEG monitoring.
Measurements
Cravings and playtime served as the primary measures of therapeutic effects. Subjective cravings were assessed using the visual analog scale (VAS) [17, 41], and the Questionnaire on Gaming Urge Brief Version (QDG-B) [42]. Objective craving was measured by the mean amplitude of the P300 wave induced by game stimuli during cue reactivity task [17]. Playtime was quantified by the amount of time participants spent playing the game each week.
Additional questionnaire assessments included the Pittsburgh Sleep Quality Index (PSQI) [43], Insomnia Severity Index (ISI) [44], Epworth Sleepiness Scale (ESS) [45], Morning and Evening Questionnaire 5 (MEQ-5) [46], Chinese Perceived Stress Scale (CPSS) [47], Brief Sensation Seeking Scale for Chinese (BSSS-C) [48], Patient Health Questionnaire-9 (PHQ-9) [49], and Beck Anxiety Inventory (BAI) [50].
Cue reactivity task
The cue reactivity task consisted of 14 sounds (7 game-related, e.g., “double kill” and 7 non-game sounds, e.g., “peaceful”) and 70 images (35 game-related and 35 non-game images). Each image was paired with a corresponding sound. The duration of each sound was approximately 1 s, and all sounds were normalized to a consistent volume of 70 dB. Each trial began with a 0.8 s fixation, followed by the presentation of an image in the center of the screen for 2 s, accompanied by the sound. After participants rated their craving levels, the next trial commenced. The paired stimuli were presented in a randomized order.
EEG data recording
The EEG signals were recorded using a 32-channel Smarting EEG system (mBrainTrain LLC, Serbia) with electrodes positioned according to the International 10-20 system. F3/F4, C3/C4, and O1/O2 were selected for online sleep monitoring, with FCz used as the online reference electrode. Two EOG channels were placed below the left and right eyes to monitor eye movements. The online EEG data were bandpass filtered from 0.5-30 Hz, with a 500 Hz sampling rate. Sleep stages were determined offline based on EEG recordings from F3, F4, C3, C4, O1, O2, and EOG for 30 s epochs, using standard criteria [51]. Total sleep time (TST) and time spent in different sleep stages (wake, sleep stages 1, 2, 3, and REM sleep) were determined by an experienced researcher for the two experiment nights.
EEG data preprocessing
The 32-channel EEG data were preprocessed using EEGLAB, a toolbox integrated within MATLAB 2021b. First, raw EEG data were filtered with a bandpass of 0.5-30 Hz. Next, bad channels were visually identified and marked. The continuous sleep EEG data were then segmented into epochs (−1-5.5 s) relative to the onset of the sound. These epochs were used for stimulus-locked event-related potentials (ERPs) and time-frequency analyses. The EEG data from the cue reactivity task was segmented into epochs (−1-2 s) for ERP analysis. Noisy EEG channels were identified through visual inspection, discarded, and interpolated using a weighted average of neighboring channels. The data were re-referenced to the average of all non-marked electrodes after removing M1 and M2. Localizer data were subjected to independent component analysis (ICA) and components associated with eye blinks and movements were identified and rejected. Four participants in the sleep groups and three participants in the awake group, with more than 30% unusable epochs during the cue reactivity task at T1 or T5. These participants were excluded from the analysis. As in other studies focusing on the coordination of SOs and spindles, both oscillations and P300 amplitude (250-500 ms) showed strong presence over central areas, making Cz the optimal target zone. Therefore, the subsequent ERP and time-frequency analyses were performed using data from Cz.
EEG date analysis
For ERPs and time-frequency analyses, we used the ERPLAB and FieldTrip toolboxes for EEG analysis in MATLAB 2021b. For ERPs, artifact-free short epochs were averaged and baseline corrected (−0.2-0 s). The P300 amplitude was calculated by averaging the amplitude within the peak time window (250-500 ms) evoked by the game auditory cue stimuli. For the time-frequency analysis of sleep EEG data, continuous wavelet transformation was applied with variance cycles (three cycles at 1 Hz in length, increasing linearly up to 15 cycles at 30 Hz) on sleep epochs (−1-6 s) to obtain power in the frequency range of 1-30 Hz, with 0.5 Hz frequency steps and a 5 ms time window. Epochs were cropped to remove edge artifacts after the time-frequency transformation. Power data were then normalized for each frequency bin and each channel by subtracting the mean power from the baseline time windows ([−0.4-−0.2 s] relative to the cue onset) and dividing by the same baseline mean power. Finally, the power data were re-segmented into 5.5 s epochs (i.e., [0.5-5 s] relative to the cue onset).
Phase-locked closed-loop auditory stimulation
The SO UP-state closed-loop auditory exposure was administered using an additional EEG recording system comprising a “Digitimer D360” EEG amplifier (Digitimer LTD, Hertfordshire, UK) and a “Power1401mk 2” high-performance data acquisition interface (Cambridge Electronic Design Ltd, UK), connected to a dedicated PC for online detection of SOs and auditory stimulation [37]. EEG signals could be viewed on the data acquisition PC using dedicated software Spike2 (Cambridge Electronic Design Ltd). Given that SOs predominantly originate in frontal regions [52, 53], the detection was based on recordings from Fpz. These recordings were referenced against linked earlobes, and bandpass-filtered in the slow-wave range (0.5-4 Hz) at a sampling rate of 200 Hz. For a signal to qualify as an SO UP-state, it needed to rise, exceeded a + 35 μV threshold, and show a positive-to-negative slope change, marking a local maximum [33]. When a positive SO peak was detected, auditory stimulation at 47 dB was triggered, after which the protocol paused for 8 s. Stimulation was paused if the participant entered REM or N1 sleep, exhibited signs of arousal or awoke. The procedure was set to conclude by researcher either (1) when approximately 300 sounds had been delivered or (2) at 03:00 a.m., whichever occurred first.
Statistical analysis
The sample size was estimated using G*Power [54]. The expected effect size of the primary outcome measure between the two groups after treatment was 0.4, with a significance level (α) of 0.05. The primary outcome was measured 6 times (baseline, after intervention, 1 week, 2 weeks, 3 weeks, and 1-month [follow-up endpoint] after initiation), with an estimated correlation of 0.5 for repeated measurements. The total sample size required to ensure 90% statistical power was calculated to be 42.
Variance in craving measures (VAS, QGU-B, P300 amplitude) and playtime were assessed using repeated-measures ANOVA with factors of group and time, applying Bonferroni correction for multiple comparisons. Independent sample t-tests were conducted to compare power across the three frequency clusters and sleep characteristics. To examine relationships between pairs of variables, Pearson’s correlation analysis and linear regression modeling were used. All p-values were two-tailed, with statistical significance set at p < 0.05. Analyses were carried out using SPSS 24 and MATLAB (2021b).
The 600 cues reactivity of each participant across the two experimental nights were averaged and divided into 20 consecutive blocks. To capture the optimal dynamic fluctuations in the power of the three main frequency clusters across the 20 consecutive blocks, we applied the locally weighted scatterplot smoothing (LOWESS) method for fitting.
Results
Basic and sleep characteristics
A total of 84 IGD participants (39 [46.43%] female; mean age [SD] = 21.09 [2.83] years) were randomly and evenly assigned to the SIG, SCG, AIG, and ACG groups. No significant differences were observed in baseline characteristics across the four groups (Table 1), and sleep characteristics were also found to have no significant differences between the SIG and the SCG groups (Table 1).
Effects of gaCET during SWS on IGD
Across sessions, the statistical analysis revealed significant effects on self-reported VAS scores (interaction effect: F(5, 36) = 2.39, p = 0.039; group main effect: F(1, 40) = 7.28, p = 0.01; time main effect: F(5, 36) = 10.40, p = 0.003) and QGU-B (interaction effect: F(5, 36) = 4.55, p = 0.003; group main effect: F(1, 40) = 5.06, p = 0.03; time main effect: F(5, 36) = 6.73, p < 0.001). Post hoc analysis indicated that within the SIG, the VAS and QGU-B scores were significantly reduced at all follow-up time points compared to T0 (T1 to T5, p < 0.05). Additionally, the SIG showed significantly lower VAS scores than the SCG at each follow-up, and lower QGU-B scores at T1, T3, and T4 (p < 0.05) (Fig. 2A and B).
A VAS score changes in SIG and SCG at baseline, post-intervention, and at 1, 2, 3 weeks, and 1 month follow-up. B QGU-B score changes in SIG and SCG at baseline, post-intervention, and at 1, 2, 3 weeks, and 1 month follow-up. C P300 mean amplitude changes in SIG and SCG at baseline, post-intervention, and at 1 month follow-up. D Playtime changes in SIG and SCG at baseline, post-intervention, and at 1, 2, 3 weeks, and 1 month follow-up. E VAS score changes in AIG and ACG at baseline, post-intervention, and at 1, 2, 3 weeks, and 1 month follow-up. F QGU-B score changes in AIG and ACG at baseline, post-intervention, and at 1, 2, 3 weeks, and 1 month follow-up. G P300 mean amplitude changes in AIG and ACG at baseline, post-intervention, and at 1-month follow-up. H Playtime changes for AIG and ACG at baseline, and at 1, 2, 3 weeks, and 1 month follow-up. *: p < 0.05; **: p < 0.01. All bar graphs display mean (±SEM). SIG, sleep intervention group; SCG, sleep control group; AIG, awake intervention group; ACG, awake control group.
Similarly, the SIG showed a significant reduction in the P300 amplitude during the cue reactivity task (the objective craving index) at T1 and T5 compared to baseline (T0), (time main effect: F(2, 36) = 5.88, p < 0.01) (Fig. 2C). Playtime in the SIG also decreased significantly at follow-up points (T2 to T5) compared with T0, and was notably lower than in the SCG at T4 and T5 (interaction effect: F(5, 36) = 4.84, p = 0.03, group main effect: F(1, 40) = 4.38, p = 0.04; time main effect: F(5, 36) = 6.79, p = 0.01) (Fig. 2D).
The effects of gaCET during awake on IGD
In both the AIG and ACG, there were no significant changes in the primary measures- VAS scores (Fig. 2E), QGU-B scores (Fig. 2F), P300 amplitude (Fig. 2G), and playtime (Fig. 2H)- from baseline (T0) either immediately post-intervention or during the follow-up period. Additionally, no significant group differences were found in these measures (p > 0.05).
EEG responses to game auditory cues during sleep and their correlation with therapeutic effects
Both game-related cues in the SIG and non-game cues in the SCG elicited significantly increased EEG power across the 2-20 Hz range during the first 1.5 s after cue presentation (Figure S1A and B). And we also examined the cue-induced ERP plots by averaging all trials of sound exposure. The onset of sound playback was marked as 0 ms. It is obvious that for both the SIG and SCG, the sound presentation coincided with the UP-state of the SO (Figure S1A and B).
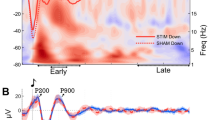
Notably, compared to the SCG, SIG exhibited a significant increase in a low-frequency power cluster (2-7 Hz at 0.2-0.5 s after cue onset, t = 3.79, p < 0.001) and an early spindle power cluster (13-17 Hz at 0.5-1.2 s after cue onset, t = 2.29, p = 0.03). Furthermore, along with a significant reduction in late spindle power (13-17 Hz at 1.8-2.6 s after cue onset; t = −2.08, p = 0.04) (Fig. 3A–D) across the two intervention nights.
A Top panel: TFR of responses to game-related cues versus non-game cues over two nights. Bottom panel: topographical plots showing game-related cue-elicited low-frequency power, early spindle power, and late spindle power. B Mean (±SEM) low-frequency power for both SIG and SCG across all subjects. C Mean (±SEM) early spindle power for both SIG and SCG across all subjects. D Mean (±SEM) late spindle power for both the SIG and the SCG across all subjects. E Correlations between power in the three frequency clusters and changes in VAS scores at T1, T2, T3, T4, and T5. F Correlations between power in the three frequency clusters and changes in QGU-B scores at T1, T2, T3, T4, and T5. G Correlations between power in the three frequency clusters and baseline playtime, along with changes in playtime at T2, T3, T4, and T5. H Correlations between power in the three frequency clusters and baseline p300 amplitude, along with changes in p300 amplitude at T0, T1, and T5. *: p < 0.05, **: p < 0.01, ***: p < 0.001. SIG, sleep intervention group; SCG, sleep control group; AIG, awake intervention group; ACG, awake control group.
We then examined correlations between cue-evoked EEG power and intervention effects in the SIG. For the subjective craving index, low-frequency power was negatively correlated with reductions in VAS scores at T4 (r = −0.5, p = 0.01) and T5 (r = −0.38, p = 0.04). Early spindle power also showed a negative correlation with decreased VAS scores at T4 (r = −0.45, p = 0.02). Similarly, both low-frequency power (r = −0.49, p = 0.01 at T4; r = −0.44, p = 0.02 at T5) and the early spindle power (r = −0.43, p = 0.02 at T4) were negatively correlated with decreased QGU-B scores. Conversely, late spindle power was positively correlated with reduced VAS scores at T1 (r = 0.38, p = 0.04) and T2 (r = 0.4, p = 0.03) (Fig. 3E and F).
The objective craving index showed a consistent correlation pattern with intervention effects in the SIG. Early spindle power was negatively correlated with changes in P300 amplitude at T1(r = −0.54, p = 0.01). Additionally, late spindle power was positively correlated with reductions in playtime (r = 0.41, p = 0.03 at T3; r = 0.43, p = 0.03 at T4) and with P300 amplitude (r = 0.41, p = 0.03 at T1; r = 0.47, p = 0.03 at T5) (Fig. 3G and H).
There was no significant correlation between the three prominent EEG power clusters and subjective and objective changes in the SCG, except for a significant positive correlation between the late spindle power and the changes in VAS scores at T1 (r = 0.48, p = 0.03) (Figure S2).
Dose-dependent EEG response across the two intervention nights
Further analyses using repeated-measures ANOVA to assess the three frequency clusters’ power in the first night and the second night between the SIG and SCG, revealed a significant main effect of the group on the power of the low-frequency power (F(1, 80) = 12.92, p = 0.001), the early spindle power (F(1, 80) = 4.95, p = 0.029), and the late spindle power (F(1, 80) = 8.57, p = 0.005). However, the interaction and intervention time effects were not statistically significant (p > 0.05) (Figure S3).
To delve deeper into the impact of the intervention time, we segmented each participant’s cue exposure trials over two nights into 20 intervention blocks. Across the 20 intervention blocks, game auditory cues evoked a descending parabolic trend in low-frequency power (Fig. 4A) and early spindle power (Fig. 4B). Notably, late spindle power exhibited a significant linear correlation with the intervention blocks (r2 = 0.31, p = 0.011) (Fig. 4C). Additionally, the slope of the linear increase in late spindle power was positively correlated with reductions in VAS scores (r = 0.54, p = 0.015) (Fig. 4D) and QGU-B scores (r = 0.49, p = 0.03) (Fig. 4E) at T1 in SIG. The y-intercept of the linear trend for late spindle power also showed a significant positive correlation with VAS score changes at T4 (r = 0.45, p = 0.04) (Fig. 4F). In contrast, for SCG, no significant linear or curvilinear changes in the three cue-evoked EEG power clusters were observed across the 20 intervention blocks (Figure S4).
A A quadratic trajectory of low-frequency power across 20 intervention blocks in the SIG. B A quadratic trend for early spindle power across 20 intervention blocks in the SIG. C A significant linear trend for late spindle power across 20 intervention blocks in the SIG. D The slope of the linear trend for late spindle power across 20 intervention blocks positively correlates with changes in VAS scores in the SIG at T1. E The slope of the linear trend for late spindle power across 20 intervention blocks positively correlates with changes in GQU-B scores in the SIG at T1. F The y-intercept of the linear trend for late spindle power across 20 intervention blocks positively correlates with changes in VAS scores in the SIG at T4. G Mean (±SEM) VAS scores for the better and weaker groups at baseline and after one month. H Mean (±SEM) QGU-B scores for the better and weaker groups at baseline and after one month. I The linear trend of the late spindle power across 20 intervention blocks in the better and the weaker groups. ***: p < 0.001. SIG, sleep intervention group.
Distinct EEG responses between better and weaker therapeutic effects
We identified two distinct clusters of therapeutic effectiveness based on VAS and QGU-B scores at the 1-month follow-up in SIG. Participants were categorized into two groups based on the reduction in QGU-B scores at 1-month post-intervention. Those with a decrease of less than 30% from baseline were assigned to the ‘weaker’ group (11 participants), while those with a decrease of more than 30% were placed in the ‘better’ group (10 participants) (Figure S5A). A significant interaction between time and group was observed, with differences between weaker and better groups (F(1, 19) = 35.51, p < 0.001 for VAS, F(1, 19) = 40.94, p < 0.001 for QGU-B) (Fig. 4G and H).
There were no significant differences between the better and weaker groups regarding basic clinical features or cue-evoked EEG power during sleep (p > 0.05, Supplementary Table S1, Figure S5B–D). Notably, the linear increase in late spindle power across the 20 intervention blocks remained significant in the better group (r2 = 0.23, p = 0.03), but not in the weaker group (r2 = 0.03, p = 0.42) (Fig. 4I).
Discussion
To our knowledge, this is the first preliminary study to evaluate the therapeutic effects of repeated auditory cue exposure during sleep for IGD. Our findings demonstrate that repeated closed-loop exposure to game-related sounds during the SO Up-state of SWS is both technically feasible and safe, resulting in a significant reduction in cravings and playtime for IGD individuals lasting at least one month. Notably, this effect was not observed during awake sessions. Mechanistic insights revealed that game-related cues elicited increased low-frequency and early spindle power, alongside reduced late spindle power, all of which were associated with decreased cravings and playtime in the SIG. Particularly, a dose-dependent escalation of late spindle power across intervention blocks was positively correlated with the therapeutic outcomes.
Our findings indicated that the same dose of sound CET intervention was more effective during sleep than wakefulness. This finding aligns with prior research on olfactory CET for disorders involving pathological memories, such as smoking [27]. Sleep is akin to an anesthetic state of unconsciousness, whereas wakefulness represents a state of consciousness [18]. According to the consciousness-dependent inhibition theory [55], CET during sleep may encounter less resistance, thereby enhancing extinction-based effects on pathological memories, compared to conscious CET reactivation while awake, which may involve greater conscious inhibition. This suggests that SWS presents a more receptive and modifiable state for the extinction of pathological memories and behaviors than wakefulness. Moreover, the lack of efficacy during wakefulness underscores the limitations of traditional CET for addiction [15, 16, 56].
Regarding neuroelectrophysiological mechanisms, game-related sounds presented during SWS significantly elevated low-frequency and early spindle power, consistent with findings from prior TMR studies [26, 57,58,59,60,61,62]. And similar oscillatory increases were associated with memory reactivation and retention [21, 33, 61, 63]. In this study, cue-elicited early spindle and low-frequency power negatively correlated with the therapeutic effects of sleep-based gaCET, suggesting they may represent subconscious reactivation of cravings. Additionally, the observed inverted U-shape pattern in these power components across intervention blocks may reflect an initial rise in subconscious cravings followed by a decline as the intervention progresses.
Aligned with previous studies [23], there was a significant reduction in late spindle power (1.8-2.6 s) following target cues exposure compared to control cues during SWS. The reduction in late spindle power in response to game-related sounds may be due to the natural spindle occurrence rhythm [64], where stronger early spindle activity initiates a refractory period (3-6 s) that reduces the likelihood of late spindles. While prior evidence has highlighted the role of cue-elicited late spindle power in memory consolidation [23]. Our findings, in line with retrieval-induced extinction theory [65, 66], in which repeated retrieval of selected game-related memories would recruit the top-down inhibitory control, specifically the late spindle power, leading to the reform of these game-related memories and the decrease in cravings. Furthermore, this cue-related activity in late spindle power reinforces the dose-dependent extinction effects and individual variability in response to auditory CET during sleep.
Our study has some limitations. First, our findings need validation through a double-blind approach to ensure robustness. Second, it remains to be seen if these findings apply to IGD associated with other game genres. Third, in order to enhance the participants’ willingness to take part in this experiment, we did not require the subjects to undergo sleep adaptation on the first night. Fourth, as some studies suggest that cue exposure during both the UP-state and DOWN-state of SO may aid in memory extinction [67], further investigation is warranted to explore the effects of cue exposure during SO DOWN-state in IGD. Additionally, further interventions targeting cue-evoked late spindle power are necessary to clarify the causative mechanisms involved.
In conclusion, our findings indicate that closed-loop repeated exposure to game-related sounds during SO UP-state in SWS presents a promising approach for reducing cravings and playtime in IGD individuals, with effects lasting at least one month. Cue-evoked late spindle power appears to play a critical role in facilitating this therapeutic outcome. This study provides research evidence for the special period and neural mechanism of unconscious memory intervention during sleep.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request and with permission of the university administration.
References
Petry NM, Rehbein F, Gentile DA, Lemmens JS, Rumpf HJ, Mößle T, et al. An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction. 2014;109:1399–406. https://doi.org/10.1111/add.12457
American Psychiatric Association, DSM-5 Task Force. (2013). Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.). American Psychiatric Publishing, Inc. https://doi.org/10.1176/appi.books.9780890425596
Gao YX, Wang JY, Dong GH. The prevalence and possible risk factors of internet gaming disorder among adolescents and young adults: systematic reviews and meta-analyses. J Psychiatr Res. 2022;154:35–43. https://doi.org/10.1016/j.jpsychires.2022.06.049
Tronson NC, Taylor JR. Addiction: a drug-induced disorder of memory reconsolidation. Curr Opin Neurobiol. 2013;23:573–80. https://doi.org/10.1016/j.conb.2013.01.022
Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–22. https://doi.org/10.1176/appi.ajp.162.8.1414
Milton AL. Drink, drugs and disruption: memory manipulation for the treatment of addiction. Curr Opin Neurobiol. 2013;23:706–12. https://doi.org/10.1016/j.conb.2012.11.008
Marissen MA, Franken IH, Blanken P, van den Brink W, Hendriks VM. Cue exposure therapy for the treatment of opiate addiction: results of a randomized controlled clinical trial. Psychother Psychosom. 2007;76:97–105. https://doi.org/10.1159/000097968
Loeber S, Croissant B, Heinz A, Mann K, Flor H. Cue exposure in the treatment of alcohol dependence: effects on drinking outcome, craving and self-efficacy. Br J Clin Psychol. 2006;45:515–29. https://doi.org/10.1348/014466505x82586
Dawe S, Powell J, Richards D, Gossop M, Marks I, Strang J, et al. Does post-withdrawal cue exposure improve outcome in opiate addiction? A controlled trial. Addiction. 1993;88:1233–45. https://doi.org/10.1111/j.1360-0443.1993.tb02146.x
Franken IH, de Haan HA, van der Meer CW, Haffmans PM, Hendriks VM. Cue reactivity and effects of cue exposure in abstinent posttreatment drug users. J Subst Abuse Treat. 1999;16:81–5. https://doi.org/10.1016/s0740-5472(98)00004-x
Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Santa Ana EJ, Saladin ME, et al. Impact of DCS-facilitated cue exposure therapy on brain activation to cocaine cues in cocaine dependence. Drug Alcohol Depend. 2013;132:195–201. https://doi.org/10.1016/j.drugalcdep.2013.02.009
Santa Ana EJ, Prisciandaro JJ, Saladin ME, McRae-Clark AL, Shaftman SR, Nietert PJ, et al. D-cycloserine combined with cue exposure therapy fails to attenuate subjective and physiological craving in cocaine dependence. Am J Addict. 2015;24:217–24. https://doi.org/10.1111/ajad.12191
Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bühler M, et al. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biol Psychiatry. 2011;69:1060–6. https://doi.org/10.1016/j.biopsych.2010.12.016
Zhang J, Chen M, Yan J, Wang C, Deng H, Wang J, et al. Effects of virtual reality-based cue exposure therapy on craving and physiological responses in alcohol-dependent patients-a randomised controlled trial. BMC Psychiatry. 2023;23:951 https://doi.org/10.1186/s12888-023-05426-z
Kiyak C, Simonetti ME, Norton S, Deluca P. The efficacy of cue exposure therapy on alcohol use disorders: a quantitative meta-analysis and systematic review. Addict Behav. 2023;139:107578 https://doi.org/10.1016/j.addbeh.2022.107578
Mellentin AI, Skøt L, Nielsen B, Schippers GM, Nielsen AS, Stenager E, et al. Cue exposure therapy for the treatment of alcohol use disorders: a meta-analytic review. Clin Psychol Rev. 2017;57:195–207. https://doi.org/10.1016/j.cpr.2017.07.006
Zhao Q, Zhang Y, Wang M, Ren J, Chen Y, Chen X, et al. Effects of retrieval-extinction training on internet gaming disorder. J Behav Addict. 2022;11:49–62. https://doi.org/10.1556/2006.2022.00006
Siegel P, Peterson BS. “All we have to fear is fear itself”: paradigms for reducing fear by preventing awareness of it. Psychol Bull. 2024;150:1118–54. https://doi.org/10.1037/bul0000437
Staresina BP, Niediek J, Borger V, Surges R, Mormann F. How coupled slow oscillations, spindles and ripples coordinate neuronal processing and communication during human sleep. Nat Neurosci. 2023;26:1429–37. https://doi.org/10.1038/s41593-023-01381-w
Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013;93:681–766. https://doi.org/10.1152/physrev.00032.2012
Cairney SA, Guttesen AÁV, El Marj N, Staresina BP. Memory consolidation is linked to spindle-mediated information processing during sleep. Curr Biol. 2018;28:948–54.e4. https://doi.org/10.1016/j.cub.2018.01.087
Lewis PA, Bendor D. How targeted memory reactivation promotes the selective strengthening of memories in sleep. Curr Biol. 2019;29:R906–r12. https://doi.org/10.1016/j.cub.2019.08.019
Liu J, Xia T, Chen D, Yao Z, Zhu M, Antony JW, et al. Item-specific neural representations during human sleep support long-term memory. PLoS Biol. 2023;21:e3002399 https://doi.org/10.1371/journal.pbio.3002399
Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007;315:1426–9. https://doi.org/10.1126/science.1138581
Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009;326:1079. https://doi.org/10.1126/science.1179013
van der Heijden AC, van der Werf YD, van den Heuvel OA, Talamini LM, van Marle HJF. Targeted memory reactivation to augment treatment in post-traumatic stress disorder. Curr Biol. 2024;34:3735–46.e5. https://doi.org/10.1016/j.cub.2024.07.019
Arzi A, Holtzman Y, Samnon P, Eshel N, Harel E, Sobel N. Olfactory aversive conditioning during sleep reduces cigarette-smoking behavior. J Neurosci. 2014;34:15382–93. https://doi.org/10.1523/jneurosci.2291-14.2014
He J, Sun HQ, Li SX, Zhang WH, Shi J, Ai SZ, et al. Effect of conditioned stimulus exposure during slow wave sleep on fear memory extinction in humans. Sleep. 2015;38:423–31. https://doi.org/10.5665/sleep.4502
Hauner KK, Howard JD, Zelano C, Gottfried JA. Stimulus-specific enhancement of fear extinction during slow-wave sleep. Nat Neurosci. 2013;16:1553–5. https://doi.org/10.1038/nn.3527
Loomis AL, Harvey EN, Hobart G. Potential rhythms of the cerebral cortex during sleep. Science. 1935;81:597–8. https://doi.org/10.1126/science.81.2111.597
Timofeev I, Chauvette S. Sleep slow oscillation and plasticity. Curr Opin Neurobiol. 2017;44:116–26. https://doi.org/10.1016/j.conb.2017.03.019
Fernandez LMJ, Lüthi A. Sleep spindles: mechanisms and functions. Physiol Rev. 2020;100:805–68. https://doi.org/10.1152/physrev.00042.2018
Ngo HV, Staresina BP. Shaping overnight consolidation via slow-oscillation closed-loop targeted memory reactivation. Proc Natl Acad Sci USA. 2022;119:e2123428119. https://doi.org/10.1073/pnas.2123428119
Hahn MA, Heib D, Schabus M, Hoedlmoser K, Helfrich RF. Slow oscillation-spindle coupling predicts enhanced memory formation from childhood to adolescence. Elife. 2020;9:e53730. https://doi.org/10.7554/eLife.53730
Schreiner T, Petzka M, Staudigl T, Staresina BP. Endogenous memory reactivation during sleep in humans is clocked by slow oscillation-spindle complexes. Nat Commun. 2021;12:3112. https://doi.org/10.1038/s41467-021-23520-2
Mölle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci USA. 2004;101:13963–8. https://doi.org/10.1073/pnas.0402820101
Ngo HV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013;78:545–53. https://doi.org/10.1016/j.neuron.2013.03.006
Schabus M, Dang-Vu TT, Heib DP, Boly M, Desseilles M, Vandewalle G, et al. The fate of incoming stimuli during NREM sleep is determined by spindles and the phase of the slow oscillation. Front Neurol. 2012;3:40. https://doi.org/10.3389/fneur.2012.00040
Destexhe A, Hughes SW, Rudolph M, Crunelli V. Are corticothalamic ‘up’ states fragments of wakefulness? Trends Neurosci. 2007;30:334–42. https://doi.org/10.1016/j.tins.2007.04.006
Tang D, Ma R, Chung P, Ho WK, Sum KR. Synergistic fields: unveiling the potential win-win relationship between esports performance and traditional sports participation. PLoS One. 2024;19:e0305880. https://doi.org/10.1371/journal.pone.0305880
Dong G, Wang L, Du X, Potenza MN. Gaming increases craving to gaming-related stimuli in individuals with internet gaming disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2:404–12. https://doi.org/10.1016/j.bpsc.2017.01.002
Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. Br J Addict. 1991;86:1467–76. https://doi.org/10.1111/j.1360-0443.1991.tb01732.x
Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Bastien CH, Vallières A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. https://doi.org/10.1016/s1389-9457(00)00065-4
Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. https://doi.org/10.1093/sleep/14.6.540
Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110.
Yang TZ, Huang HT. An epidemiological study on stress among urban residents in social transition period. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24:760–4.
Chen X, Li F, Nydegger L, Gong J, Ren Y, Dinaj-Koci V, et al. Brief sensation seeking scale for chinese - cultural adaptation and psychometric assessment. Pers Individ Dif. 2013;54:604–9. https://doi.org/10.1016/j.paid.2012.11.007
Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
Fydrich T, Dowdall D, Chambless DL. Reliability and validity of the beck anxiety inventory. J Anxiety Disord. 1992;6:55–61. https://doi.org/10.1016/0887-6185(92)90026-4
Wolpert EA. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Arch Gen Psychiatry. 1969;20:246–7. https://doi.org/10.1001/archpsyc.1969.01740140118016
Chauvette S, Volgushev M, Timofeev I. Origin of active states in local neocortical networks during slow sleep oscillation. Cereb Cortex. 2010;20:2660–74. https://doi.org/10.1093/cercor/bhq009
Massimini M, Huber R, Ferrarelli F, Hill S, Tononi G. The sleep slow oscillation as a traveling wave. J Neurosci. 2004;24:6862–70. https://doi.org/10.1523/jneurosci.1318-04.2004
Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. https://doi.org/10.3758/bf03193146
Tal A, Schechtman E, Caughran B, Paller KA, Davachi L. The reach of reactivation: Effects of consciously triggered versus unconsciously triggered reactivation of associative memory. Proc Natl Acad Sci USA. 2024;121:e2313604121. https://doi.org/10.1073/pnas.2313604121
Conklin CA, Tiffany ST. Cue-exposure treatment: time for change. Addiction. 2002;97:1219–21. https://doi.org/10.1046/j.1360-0443.2002.00205.x
Wislowska M, Klimesch W, Jensen O, Blume C, Schabus M. Sleep-specific processing of auditory stimuli is reflected by alpha and sigma oscillations. J Neurosci. 2022;42:4711–24. https://doi.org/10.1523/jneurosci.1889-21.2022
Andrillon T, Poulsen AT, Hansen LK, Léger D, Kouider S. Neural markers of responsiveness to the environment in human sleep. J Neurosci. 2016;36:6583–96. https://doi.org/10.1523/jneurosci.0902-16.2016
Nicolas J, King BR, Levesque D, Lazzouni L, Coffey E, Swinnen S, et al. Sigma oscillations protect or reinstate motor memory depending on their temporal coordination with slow waves. Elife. 2022;11:e73930. https://doi.org/10.7554/eLife.73930
Rakowska M, Abdellahi MEA, Bagrowska P, Navarrete M, Lewis PA. Long term effects of cueing procedural memory reactivation during NREM sleep. Neuroimage. 2021;244:118573. https://doi.org/10.1016/j.neuroimage.2021.118573
Schreiner T, Griffiths BJ, Kutlu M, Vollmar C, Kaufmann E, Quach S, et al. Spindle-locked ripples mediate memory reactivation during human NREM sleep. Nat Commun. 2024;15:5249. https://doi.org/10.1038/s41467-024-49572-8
Xia T, Chen D, Zeng S, Yao Z, Liu J, Qin S, et al. Aversive memories can be weakened during human sleep via the reactivation of positive interfering memories. Proc Natl Acad Sci USA. 2024;121:e2400678121. https://doi.org/10.1073/pnas.2400678121
Geva-Sagiv M, Mankin EA, Eliashiv D, Epstein S, Cherry N, Kalender G, et al. Augmenting hippocampal-prefrontal neuronal synchrony during sleep enhances memory consolidation in humans. Nat Neurosci. 2023;26:1100–10. https://doi.org/10.1038/s41593-023-01324-5
Antony JW, Piloto L, Wang M, Pacheco P, Norman KA, Paller KA. Sleep spindle refractoriness segregates periods of memory reactivation. Curr Biol. 2018;28:1736–43.e4. https://doi.org/10.1016/j.cub.2018.04.020
Wimber M, Alink A, Charest I, Kriegeskorte N, Anderson MC. Retrieval induces adaptive forgetting of competing memories via cortical pattern suppression. Nat Neurosci. 2015;18:582–9. https://doi.org/10.1038/nn.3973
Anderson MC, Bjork RA, Bjork EL. Remembering can cause forgetting: retrieval dynamics in long-term memory. J Exp Psychol Learn Mem Cogn. 1994;20:1063–87. https://doi.org/10.1037//0278-7393.20.5.1063
van der Heijden AC, van den Heuvel OA, van der Werf YD, Talamini LM, van Marle HJF. Sleep as a window to target traumatic memories. Neurosci Biobehav Rev. 2022;140:104765. https://doi.org/10.1016/j.neubiorev.2022.104765
Acknowledgements
This work was supported in part by the STI2030-Major Projects (2021ZD0202100, 2021ZD0200801) and the National Natural Science Foundation of China (82130040, 82288101).
Author information
Authors and Affiliations
Contributions
X-QY, JS and YS conceived and designed the study. X-QY, Y-XS, W-YL and Y-CH performed the experiments. X-QY, YS, T-YJ and J-FL analyzed and interpreted the data. X-QY, Y-XS, W-YL, Y-CH, T-YJ, J-FL, LL, YS and JS wrote and revised the manuscript. JS provided the research funds. All the authors contributed to the article and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, X., Song, Y., Liu, W. et al. Efficacy and mechanisms of repeated closed-loop auditory exposure during slow-wave sleep for internet gaming disorder. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-02995-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-02995-1