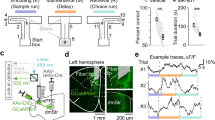
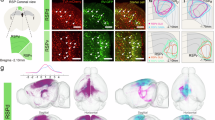
Abstract
The dorsal subiculum (dSub) and medial mammillary body (MM) are essential components of the extended hippocampal system, heavily implicated in spatial memory processes. However, the exact function of the prominent dSub-to-MM projection has remained ambiguous. Here, integrating c-fos mapping, fiber photometry, optogenetic manipulations and behavioral paradigms, we found that the monosynaptic connection between dSub and MM, rather than collaterals to other regions, is not only involved in spatial memory retrieval but also highly vulnerable to perturbations on activity level in both directions. In addition, we mapped the single neuron projectome of MM-projecting dSub neurons using fluorescence micro-optical sectioning tomography (fMOST). Our study unveiled that the individual dSub neuron extend efferent connections to the bilateral MM neurons, while also exhibiting substantial collateral projections to the retrosplenial cortex (RSP) and entorhinal (ENT). Our findings shed light on previously unknown subtypes and organizational principles of projection neurons in the afferent circuitry of the MM, thereby elucidating the circuit mechanism underlying the contribution of the dSub-MM circuit to spatial memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data that support the findings of this study are provided in the article and its supplementary information files and source data. Raw data for neuron reconstruction is available at http://atlas.brainsmatics.org/a/sun2112. Source data are provided with this paper. Additional information about this paper are available from the corresponding author upon reasonable request.
Code availability
The code for image processing is available in previous studies [22, 51], which can be found from http://atlas.brainsmatics.org/a/zhong2019.
References
Winnubst J, Bas E, Ferreira TA, Wu Z, Economo MN, Edson P, et al. Reconstruction of 1000 projection neurons reveals new cell types and organization of long-range connectivity in the mouse brain. Cell. 2019;179:268–81.e13.
Umaba R, Kitanishi T, Mizuseki K. Monosynaptic connection from the subiculum to medial mammillary nucleus neurons projecting to the anterior thalamus and Gudden’s ventral tegmental nucleus. Neurosci Res. 2021;171:1–8.
Aggleton JP, Christiansen K. The subiculum: the heart of the extended hippocampal system. Prog Brain Res. 2015;219:65–82.
Kitanishi T, Umaba R, Mizuseki K. Robust information routing by dorsal subiculum neurons. Sci Adv. 2021;7:eabf1913.
Roy DS, Kitamura T, Okuyama T, Ogawa SK, Sun C, Obata Y, et al. Distinct neural circuits for the formation and retrieval of episodic memories. Cell. 2017;170:1000–12.e19.
Sun Y, Jin S, Lin X, Chen L, Qiao X, Jiang L, et al. CA1-projecting subiculum neurons facilitate object–place learning. Nat Neurosci. 2019;22:1857–70.
Thierry M, Boluda S, Delatour B, Marty S, Seilhean D, Brainbank Neuro-CEB Neuropathology Network. et al. Human subiculo-fornico-mamillary system in Alzheimer’s disease: tau seeding by the pillar of the fornix. Acta Neuropathol. 2020;139:443–61.
Mathiasen ML, Louch RC, Nelson AD, Dillingham CM, Aggleton JP. Trajectory of hippocampal fibres to the contralateral anterior thalamus and mammillary bodies in rats, mice, and macaque monkeys. Brain Neurosci Adv. 2019;3:2398212819871205.
Vann SD. Dismantling the Papez circuit for memory in rats. eLife. 2013;2:e00736.
Kinnavane L, Vann SD, Nelson AJD, O’Mara SM, Aggleton JP. Collateral Projections innervate the mammillary bodies and retrosplenial cortex: a new category of hippocampal cells. eNeuro. 2018;5:ENEURO.0383-17.
Nelson AJD, Vann SD. Mammilliothalamic tract lesions disrupt tests of visuo-spatial memory. Behav Neurosci. 2014;128:494–503.
Gutiérrez-Guzmán BE, Hernández-Pérez JJ, Olvera-Cortés ME. Serotonergic modulation of septo-hippocampal and septo-mammillary theta activity during spatial learning, in the rat. Behav Brain Res. 2017;319:73–86.
Santı́n LJ, Rubio S, Begega A, Arias JL. Effects of mammillary body lesions on spatial reference and working memory tasks. Behav Brain Res. 1999;102:137–50.
Dillingham CM, Milczarek MM, Perry JC, Frost BE, Parker GD, Assaf Y, et al. Mammillothalamic disconnection alters hippocampocortical oscillatory activity and microstructure: implications for diencephalic amnesia. J Neurosci. 2019;39:6696.
Zhang J, Long B, Li A, Sun Q, Tian J, Luo T, et al. Whole-brain three-dimensional profiling reveals brain region specific axon vulnerability in 5xFAD mouse model. Front Neuroanat. 2020;14:1–14.
Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, et al. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–42.
Vann SD, Erichsen JT, O’Mara SM, Aggleton JP. Selective disconnection of the hippocampal formation projections to the mammillary bodies produces only mild deficits on spatial memory tasks: implications for fornix function. Hippocampus. 2011;21:945–57.
Kocsis B, Vertes R. Characterization of neurons of the supramammillary nucleus and mammillary body that discharge rhythmically with the hippocampal theta rhythm in the rat. J Neurosci. 1994;14:7040–52.
Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5:35–44.
Żakowski W, Braszka Ł, Zawistowski P, Orzeł-Gryglewska J, Jurkowlaniec E. Inactivation of the medial mammillary nucleus attenuates theta rhythm activity in the hippocampus in urethane-anesthetized rats. Neurosci Lett. 2017;645:19–24.
Qiu L, Zhang B, Gao Z. Lighting up neural circuits by viral tracing. Neurosci Bull. 2022;38:1383–96.
Gong H, Xu D, Yuan J, Li X, Guo C, Peng J, et al. High-throughput dual-colour precision imaging for brain-wide connectome with cytoarchitectonic landmarks at the cellular level. Nat Commun. 2016;7:12142.
Yang X, Jiang T, Liu L, Zhao X, Yu X, Yang M, et al. Observing single cells in whole organs with optical imaging. J Innov Opt Health Sci. 2022;16:2330002.
Sun Q, Zhang J, Li A, Yao M, Liu G, Chen S, et al. Acetylcholine deficiency disrupts extratelencephalic projection neurons in the prefrontal cortex in a mouse model of Alzheimer’s disease. Nat Commun. 2022;13:998.
Etter G, van der Veldt S, Manseau F, Zarrinkoub I, Trillaud-Doppia E, Williams S. Optogenetic gamma stimulation rescues memory impairments in an Alzheimer’s disease mouse model. Nat Commun. 2019;10:5322.
Trimper JB, Galloway CR, Jones AC, Mandi K, Manns JR. Gamma oscillations in rat hippocampal subregions dentate gyrus, CA3, CA1, and subiculum underlie associative memory encoding. Cell Rep. 2017;21:2419–32.
Zhang C, Zhu H, Ni Z, Xin Q, Zhou T, Wu R, et al. Dynamics of a disinhibitory prefrontal microcircuit in controlling social competition. Neuron. 2022;110:516–31.e6.
Li Y, Gong H, Yang X, Yuan J, Jiang T, Li X, et al. TDat: an efficient platform for processing petabyte-scale whole-brain volumetric images. Front Neural Circuits. 2017;11:51.
Zhou H, Li S, Li A, Huang Q, Xiong F, Li N, et al. GTree: an open-source tool for dense reconstruction of brain-wide neuronal population. Neuroinformatics. 2021;19:305–17.
Fei F, Wang X, Xu C, Shi J, Gong Y, Cheng H, et al. Discrete subicular circuits control generalization of hippocampal seizures. Nat Commun. 2022;13:5010.
Chen S, He L, Huang AJY, Boehringer R, Robert V, Wintzer ME, et al. A hypothalamic novelty signal modulates hippocampal memory. Nature. 2020;586:270–4.
Vetere G, Xia F, Ramsaran AI, Tran LM, Josselyn SA, Frankland PW. An inhibitory hippocampal–thalamic pathway modulates remote memory retrieval. Nat Neurosci. 2021;24:685–93.
Vann SD. Re-evaluating the role of the mammillary bodies in memory. Neuropsychologia. 2010;48:2316–27.
Dillingham CM, Milczarek MM, Perry JC, Vann SD. Time to put the mammillothalamic pathway into context. Neurosci Biobehav Rev. 2021;121:60–74.
Vann SD, Nelson AJD. The mammillary bodies and memory: more than a hippocampal relay. Progress in brain research. 2015;219:163–85.
Cembrowski MS, Phillips MG, DiLisio SF, Shields BC, Winnubst J, Chandrashekar J, et al. Dissociable structural and functional hippocampal outputs via distinct subiculum cell classes. Cell. 2018;174:1036.
Kim Y, Yang GR, Pradhan K, Venkataraju KU, Bota M, García del Molino LC, et al. Brain-wide maps reveal stereotyped cell-type-based cortical architecture and subcortical sexual dimorphism. Cell. 2017;171:456–69.e22.
Mickelsen LE, Flynn WF, Springer K, Wilson L, Beltrami EJ, Bolisetty M, et al. Cellular taxonomy and spatial organization of the murine ventral posterior hypothalamus. eLife. 2020;9:e58901.
Aggleton JP, Nelson AJD, O’Mara SM. Time to retire the serial Papez circuit: Implications for space, memory, and attention. Neurosci Biobehav Rev. 2022;140:104813.
Dillingham CM, Frizzati A, Nelson AJD, Vann SD. How do mammillary body inputs contribute to anterior thalamic function? Neurosci Biobehav Rev. 2015;54:108–19.
Gail Canter R, Huang W-C, Choi H, Wang J, Ashley Watson L, Yao CG, et al. 3D mapping reveals network-specific amyloid progression and subcortical susceptibility in mice. Commun Biol. 2019;2:360.
Mathiasen ML, Amin E, Nelson AJD, Dillingham CM, O’Mara SM, Aggleton JP. Separate cortical and hippocampal cell populations target the rat nucleus reuniens and mammillary bodies. Eur J Neurosci. 2019;49:1649–72.
Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–404.
Tingley D, Buzsáki G. Transformation of a spatial map across the hippocampal-lateral septal circuit. Neuron. 2018;98:1229–42.e5.
Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10:792–802.
Alexander A, Soltesz I. Hippogate: a break-in from entorhinal cortex. Nat Neurosci. 2016;19:530–2.
van Groen T, Kadish I, Wyss JM. Retrosplenial cortex lesions of area Rgb (but not of area Rga) impair spatial learning and memory in the rat. Behav Brain Res. 2004;154:483–91.
Vann SD, Aggleton JP. Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci. 2005;119:1682–6.
Tian J, Ren M, Zhao P, Luo S, Chen Y, Xu X, et al. Dissection of the long-range projections of specific neurons at the synaptic level in the whole mouse brain. Proc Natl Acad Sci USA. 2022;119:e2202536119.
Aggleton JP, O’Mara SM. The anterior thalamic nuclei: core components of a tripartite episodic memory system. Nat Rev Neurosci. 2022;23:505–16.
Zhong Q, Li A, Jin R, Zhang D, Li X, Jia X, et al. High-definition imaging using line-illumination modulation microscopy. Nat Methods. 2021;18:309–15.
Acknowledgements
We thank the members of HUST-Suzhou Institute for Brainsmatics for help with data acquisition on the neuron reconstruction. This work was financially supported by STI2030-Major Projects (Nos. 2022ZD0205201 and 2022ZD0206500), National Natural Science Foundation of China (Nos. 32400850), Hainan Natural Science Foundation of China (Nos. 822QN298), China Postdoctoral Science Foundation (Nos. 2022M710989), Hainan Postdoctoral Science Foundation (Nos. 2022–32) and Innovational Fund for Scientific and Technological Personnel of Hainan Province (KJRC2023A03).
Author information
Authors and Affiliations
Contributions
XL, HG, JZ and QL conceived and designed the study. JZ and MY performed most of the experiments and analyzed the data. TJ performed the whole-brain data acquisition. AL performed the imaging processing and neuron reconstruction. JZ, XL, MH, HG and QL wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations of the Animal Care and Use Committee of Hainan University (HNUAUCC-2022-00032). The informed consent was obtained from all participants. There were no identifiable images from human research participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Yao, M., Jiang, T. et al. A dorsal subiculum-medial mammillary body pathway for spatial memory. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03087-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-03087-w