Abstract
The role of the endocannabinoid system (ECS) in major depressive disorder (MDD) is under-investigated despite reports of increased activity and/or concentration of fatty acid amide hydrolase (FAAH), a key ECS enzyme, in fronto-limbic brain regions in some animal models of depressive behavior. We hypothesized that [11C]CURB λk3, an index of FAAH density, would be elevated in the prefrontal cortex, hippocampus, and anterior cingulate cortex in major depressive episodes of MDD compared to healthy controls. Fifteen unmedicated MDD participants and 15 age- and sex-matched healthy controls underwent [11C]CURB positron emission tomography and FAAH genotyping. Psychological tests of depressive severity, apathy, and anxiety were administered and measurements were assessed as covariates in exploratory analyses. No significant group differences in [11C]CURB λk3 were observed between MDD participants and controls (F1,27 = 0.32; p = 0.58). A mixed effects model revealed that Marin Apathy Evaluation Scale scores in the MDD group had a significant main effect on [11C]CURB λk3 binding across the collective regions of medial prefrontal cortex, orbitofrontal cortex, anterior cingulate cortex, ventral striatum, and midbrain (F1,11 = 6.75; p = 0.02). Depressive severity and anxiety did not have a significant relationship to [11C]CURB λk3 binding. The relationship of greater fronto-limbic [11C]CURB λk3 to greater apathy along with the metabolic role of FAAH in the ECS, the latter which supports maintaining feelings of interest, initiative, and motivation, has important implications for the pathophysiology of apathy in MDD.
Similar content being viewed by others
Introduction
Major depressive disorder (MDD) affects approximately 280 million people and is one of the leading causes of disability worldwide [1, 2]. Fifty percent of cases are treatment-resistant to first- and second-line monoamine-targeting antidepressants, such as selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors; and while esketamine and ketamine help some patients with MDD, approximately 30% of cases do not respond to these medications [3, 4]. A plausible explanation for treatment resistance is that MDD is heterogeneous and that there are as yet undetermined pathologies that remain untargeted by current pharmacological approaches. Hence, identifying such pathologies and eventually developing novel, targeted therapeutics may address these shortcomings.
Accumulating evidence suggests that dysregulation of the endocannabinoid system (ECS), which has functional roles in mood, motivation, appetite control, and memory processing, may contribute to depressive symptoms [5, 6]. Fatty acid amide hydrolase (FAAH) is an ECS enzyme that metabolizes several fatty acid amides; and is the principal metabolic enzyme of N-arachidonoylethanolamine (AEA, or anandamide), which is an endogenous ligand of cannabinoid 1 (CB1) receptors [7]. In some preclinical models of depressive behavior, FAAH activity, mRNA, and protein are increased in regions that participate in affect and motivation, such as the prefrontal cortex (PFC), hippocampus, and striatum [8, 9]. In humans, the CB1 receptor antagonist, rimonabant, is associated with an increased risk of developing or exacerbating depressive symptoms [10]. Post-mortem studies of MDD patients, albeit still in a preliminary phase of investigation, report increased neural CB1 receptor mRNA and protein in the PFC, consistent with a model of elevated FAAH activity, reduced AEA; and less stimulation of CB1 receptors with their upregulation [11, 12]. There is also considerable interest in the therapeutics of FAAH inhibition. For example, AEA-CB1 signaling has been associated with anti-depressive and anxiolytic-like effects as well as stress in rodents [13,14,15]. In response to stressors, such as in the chronic unpredictable stress [16] or the chronic mild stress models of depression [8], FAAH can initiate the degradation of AEA, thus reducing AEA-CB1 signaling. Furthermore, genetic knockout or pharmacological inhibition of FAAH was found to reduce depressive-like behavior in rodents [17, 18]. Presently, a key gap in the literature is an absence of investigations of brain FAAH in major depressive episodes (MDE) of MDD.
Positron emission tomography (PET) imaging with [11C-carbonyl]6-hydroxy-[1,1′-biphenyl]-3-yl cyclohexylcarbamate ([11C]CURB), is the most advanced method to detect λk3, an index of brain FAAH levels in vivo [19]. [11C]CURB has high selectivity and high brain uptake; and radiolabelled metabolites do not cross the blood brain barrier [20]. [11C]CURB is modelled with an irreversible two-tissue compartment kinetic model; and there is no effect of blood flow on λk3 [21]. FAAH levels are associated with FAAH activity, as demonstrated by paradigms that influence FAAH levels, such as the rs324420 variant and models of under- or overexpression of the FAAH gene [22,23,24].
Several preclinical models of depressive behavior have reported elevated FAAH within fronto-limbic brain regions, and FAAH gene over-expression has led to depressive-like behavior and a reduction in AEA [25, 26]. Evidence of FAAH elevation in fronto-limbic regions may translate to humans with MDD; thus, the main objective of this study was to compare [11C]CURB λk3 in the PFC, anterior cingulate cortex (ACC), and hippocampus in MDD with a current MDE versus healthy controls. Our primary hypothesis is that [11C]CURB λk3 will be elevated in the MDD group relative to controls. These regions were chosen as our primary brain regions of interest (ROI) because these regions are known to have a high density of FAAH [27] and also participate in emotion and affect regulation [28, 29].
λk3 is the measured index of FAAH density. It is derived from a two-compartment modeling solution in brain tissue of [11C]CURB transfer from arterial plasma in which one compartment represents free and non-specific binding and the other specific binding in which there is negligible transfer from specific binding to free and non-displaceable binding compartments. k3 is the imaging agent transfer from the free and non-specifically bound compartment to the specific binding compartment and λ is the ratio of free and non-specifically bound imaging agent to arterial blood at equilibrium. λk3 is also a desirable measure because it is not related to blood flow, previously demonstrated with arterial spin labelling a property attributable to a k3/k2 ratio of approximately 0.55 in contrast to most other irreversible PET imaging agents in which k3>>k2 [21].
Given that the endocannabinoid system is known to influence response to stress, mood regulation, level of anxiety and motivation in animals involving a substantial number of behaviors [15, 25, 26, 30,31,32], in our study design we prioritized behaviors that had greater demonstration in humans, were more specifically related to alterations in available FAAH level and/or more present in MDD. Notable examples within this scope include reports that CB1 antagonism may induce depressive symptoms [10], FAAH inhibitor JNJ-42165279 had anxiolytic effects in social anxiety disorder [33] and cannabis use may be associated with apathy [34]. Another set of notable findings is that loss of FAAH from destabilization related to a single nucleotide polymorphism (rs324420) in humans is associated with reduced anxiety [35], and increased behaviors fostered by greater motivation [36]. Apathy and anxiety are present in the majority of MDEs [37, 38].
We note that apathy has been succinctly defined as a lack of motivation, but more recent descriptions include a sustained quantitative reduction of goal directed activity accompanied by diminished emotional reactions and/or social engagement [39, 40]. Apathy is important in MDD as it is present in the majority of cases [38, 41] but it is not used as diagnostic criterion due to lack of clinical specificity [42]. The Marin Apathy Evaluation Scale is a commonly applied scale to assess apathy with excellent internal consistency, reliability, and is elevated in populations reporting apathy such as those with MDEs [38]. Anhedonia is part of the definition of an MDE and relates to apathy since it is a loss of interest or pleasure in previously rewarding activities [43].
Considering the above evidence and the functional circuits involved in depression, anxiety, and apathy, there are several exploratory hypotheses in the present study. First, we hypothesized that depressive severity has a positive association with [11C]CURB λk3 in brain regions of the MDD group implicated in regulating depressed mood, including the PFC, ACC, and hippocampus. Second, in the MDD group, we hypothesized that anxiety is positively associated with [11C]CURB λk3 in brain regions related to such, including the orbitofrontal cortex (OFC), ACC, hippocampus, insula, and amygdala. Third, in the MDD group, we anticipated that apathy would be positively associated with [11C]CURB λk3 in brain regions whose altered function is implicated in apathy, including the medial prefrontal cortex (mPFC), OFC, ACC, ventral striatum, and midbrain (including the substantia nigra and ventral tegmental area).
Materials and methods
All participants provided written informed consent before initiating the study procedures. All components of the study were approved by Health Canada and the Research Ethics Board of the Centre for Addiction and Mental Health (CAMH) in Toronto, Ontario, Canada.
Participants
Fifteen MDD participants with a current MDE and 15 age- and sex-matched healthy controls completed the study protocol. All participants were recruited from the local community in Toronto, Canada between January 2021 and July 2023. All psychiatric diagnoses were verified with the Structured Clinical Interview for DSM-5 – Research Version (SCID-5-RV) by trained raters [44], and then reviewed and confirmed by a psychiatrist (NJK). Individuals with MDD were included if they scored greater than or equal to 17 on the 17-item Hamilton Depression Rating Scale (HDRS) [45]. Exclusion criteria for the MDD participants included use of psychotropic medication in the past six weeks; history of bipolar or schizophrenia spectrum disorder; antisocial or borderline personality disorder; and history of alcohol or substance abuse in the past 12 months, as confirmed by the SCID-5-RV [44]. Healthy controls were matched to MDD participants by age within 5 years, sex, and rs324420 genotype [23], the latter which influences [11C]CURB λk3 (see FAAH Genotyping later in methods section).
Exclusion criteria for healthy controls included a score greater than seven (which represents the threshold for health) on the HDRS or any lifetime history of psychiatric disorders or psychotropic medication use. Neurological illness, head trauma, a positive drug screen for drugs of abuse, pregnancy in females, and contraindications to safe magnetic resonance imaging (MRI) scanning also precluded participation in both MDD and healthy cohorts.
Depressive severity, apathy, and anxiety were assessed with the 17-item HDRS [45], the Marin Apathy Evaluation Scale Self Version (MAES) [38], and the Hamilton Anxiety Rating Scale (HAM-A) respectively [46].
Image acquisition and analysis
The radiosynthesis of [11C]CURB was completed as previously described by our laboratories [19]. Participants wore a thermoplastic mask to reduce head movement for the duration of the PET scan. Each participant completed one [11C]CURB PET scan in a Discovery MI PET-CT scanner (General Electric, Milwaukee, WI, USA) following an intravenous injection of 370 ± 40 MBq (10 ± 1 mCi) of [11C]CURB [47]. Brain radioactivity was computed during sequential frames of increasing duration, and the scan time was 60 min to allow sufficient time for λk3 to stabilize [21]. Due to the widespread distribution of FAAH in the brain, there is no adequately large reference region to quantify [11C]CURB, necessitating the measurement of the arterial input function [21, 26]. Following the [11C]CURB injection, arterial blood was sampled continuously for the first 22.5 min with an automatic blood sampling system (ABSS Model PBS-101, Veenstra Instruments, Joure, Netherlands) and manual samples of 4–10 mL were also collected at 3, 7, 12, 20, 30, 45, and 60 min. Radioactivity of whole blood and plasma aliquots from the manual and automatic samples were measured in a gamma counter. The whole blood-to-plasma radioactivity ratio, interpolated by a biexponential function and parent plasma fraction fit with a Hill function, was applied to create the metabolite-corrected arterial input function [21].
In addition, each participant completed a standard T1-weighted brain MRI scan (TR = 6.7 ms, TE = 3 ms, TI = 650 ms, flip angle=8°, field of view = 230 × 230 mm, matrix size = 256 × 256 × 200, voxel size=0.9 mm), acquired on a Discovery MR750 3 T MRI scanner (General Electric, Milwaukee, WI, USA) for ROI delineation. The ROIs were transformed onto the PET image from the co-registration parameters of the T1 MRI to the motion-corrected PET image (PMOD Technologies LLC, RRID: SCR_016547, version 4.202). Time activity curves (TACs) were extracted from each ROI, and an irreversible two-tissue compartment model was used, along with the arterial input function, to fit the TACs, generating the composite rate constant λk3 (λ = K1/k2) to quantify [11C]CURB binding as previously validated [21].
FAAH polymorphism genotyping
Baseline FAAH protein levels are known to be affected by a single nucleotide polymorphism in the human FAAH gene (rs324420) that substitutes a cytosine for an adenine nucleoside (C385A), which results in the substitution of a proline to a threonine at amino acid position 129 (P129T). Relative to the C/C genotype, those homozygous or heterozygous for the A allele have similarly reduced [11C]CURB binding (λk3) in the brain [23]. The FAAH rs324420 variant was genotyped for all participants according to the manufacturer’s directions for a TaqMan SNP Genotyping assay (ID C_1897306_10; Life Technologies, Burlington, ON, Canada) on a ViiA7 instrument (Life Technologies, Burlington, ON, Canada) using 20 ng total genomic DNA template, Perfecta FastMix II (Quantabio, Beverly, MA, USA), in a total reaction volume of 10 µL.
Statistical analysis
To analyze [11C]CURB λk3 between groups, a linear mixed effects model was used with diagnosis and genotype included as fixed factors, regional [11C]CURB λk3 as the repeated within-subjects measure, and participant as a random effect. The prioritized group comparison included the subregions of the PFC, ACC, and hippocampus. A secondary linear mixed effects model compared groups across regional [11C]CURB λk3 in a broader range of grey matter regions throughout the brain, including the amygdala, ventral striatum, putamen, dorsal caudate, temporal cortex, occipital cortex, insula, thalamus, substantia nigra, pons, and cerebellum. To assess our exploratory hypotheses, three linear mixed effects models were applied to the MDD sample, with either HDRS, MAES, or HAM-A total scores as a covariate, genotype as a fixed factor, brain region as a repeated measure, and participant as a random effect. For the primary outcome measure, a threshold of p = 0.05 was applied for significance. Our three exploratory outcomes were effect of HDRS, effect of MAES, and effect of HAM-A on λk3 in a group of regions creating three additional analyses. However, none of these analyses are independent: Effect of diagnosis is related to effect of HDRS as diagnosis corresponds to a threshold of HDRS. Both the MAES and HAM-A can be correlated with total severity of HDRS in large samples. So, the latter measures, reflecting approximately two analyses were given a meaningful significance threshold of p = 0.025. In addition, Partial Pearson correlation coefficients were utilized to analyze [11C]CURB λk3 and symptom measures in each individual brain ROI, controlling for genotype.
Results
Demographics
Fifteen MDD participants and 15 healthy controls were included in the study (Table 1). There were 11 females (73%) and 4 males (27%) in each group. The mean age was 26.6 years (range: 19 to 47 years) for MDD participants and 25.3 years (range: 19 to 43 years) for healthy controls. There were no significant differences between groups regarding race, body mass index, or years of education. Ten participants in each group (67%) carried the C/C genotype and five participants in each group (33%) carried the heterozygous A/C genotype. All participants were free from cannabis use for at least four weeks prior to study enrolment as verified by self-report and urine drug screen. There were no group differences in radiotracer injected dose (7.36 ± 6.39 vs. 8.92 ± 9.33 nmol; t = -0.53; p = 0.60) and specific activity at the time of injection (74.5 ± 42.4 vs. 75.0 ± 45.4 TBq/mmol; t = -0.03, p = 0.98) (Table S1).
Comparison of [11C]CURB λk 3 between MDD and healthy
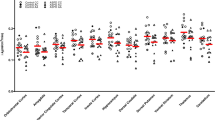
Contrary to our primary hypothesis, a linear mixed effects model demonstrated no significant group effect of [11C]CURB λk3 between MDD participants and healthy controls in the repeated measures of PFC, ACC, and hippocampus (F1,27 = 0.32, p = 0.58; Fig. 1). There was no significant effect of sex on [11C]CURB λk3 (F1,27 = 0.17, p = 0.69). As an additional analysis we applied the same linear mixed effects model and found no significant group effect of [11C]CURB λk3 between MDD participants and healthy controls across all brain ROIs (F1,27 = 0.33, p = 0.57; Table S2). An additional, exploratory, linear mixed effects model evaluating effect of rs324420 genotype across the sample found that C/C carriers had significantly higher λk3 values in the primary regions of interest as compared to A/C (F1,28 = 5.97, p = 0.02).
There was no significant group effect of [11C]CURB λk3 between MDD participants and healthy controls (F1,27 = 0.32, p = 0.58). Red bars indicate means, adjusted for genotype. dlPFC dorsolateral prefrontal cortex, vlPFC ventrolateral prefrontal cortex, mPFC medial prefrontal cortex, OFC orbitofrontal cortex, ACC anterior cingulate cortex.
Relationship of psychological measures to [11C]CURB λk 3
MAES scores in the MDD group were a significant covariate of [11C]CURB λk3 binding in the linear mixed effects model that included repeated measures in brain regions related to apathy, sampling the mPFC, OFC, ACC, ventral striatum, substantia nigra, and remainder of midbrain (F1,11 = 6.81, p = 0.02; Table 2) which is consistent with the correlation between MAES scores and a composite region of [11C]CURB λk3 binding derived from these individual regions (r = 0.60, p = 0.02; Fig. 2). Correlation coefficients between MAES scores and [11C]CURB λk3 binding in individual regions were higher in mPFC, OFC, ventral striatum, and substantia nigra (r = 0.60 to 0.70, p = 0.01 to 0.03; Fig. 3, Table 2), but less so in ACC (r = 0.33, p = 0.27) and midbrain (r = 0.51, p = 0.08; Fig. 3, Table 2). To assess the specificity of the relationship between λk3 and the ventral striatum, substantia nigra, OFC, and mPFC, an exploratory linear mixed effects model was completed evaluating the interaction between region and covariation of the MAES including these same regions as well as several regions not directly implicated in apathy, such as the occipital cortex and temporal cortex. The interaction between region and covariation of MAES was significant (F6,24 = 5.15, p = 0.002). Correlations with subscales of the MAES including cognitive (lack of interest), behavioral (lack of action) and emotional (lack of emotion) were explored and higher correlations were observed in the medial prefrontal cortex, the orbitofrontal cortex, ventral striatum and substantia nigra in relation to the cognitive subscale were noted (Table S3). The mean MAES was 41, and the majority of the MDD sample (n = 12) had a MAES total score above 34, indicative of substantial apathy uncommon in the midst of health. Total HDRS score was not a significant covariate of [11C]CURB λk3 binding in the PFC, ACC, and hippocampus (F1,11 < 0.01, p = 0.97; Table 2). HAM-A score was also not a significant covariate of [11C]CURB λk3 binding in brain regions related to anxiety in the MDD group, including the OFC, ACC, hippocampus, insula, and amygdala (F1,11 = 0.75, p = 0.49; Table 2).
MDD participants who had higher MAES scores also had greater [11C]CURB λk3 binding in the medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC), ventral striatum, and substantia nigra (r = 0.60 to 0.70, p = 0.01 to 0.03) but less so in the anterior cingulate cortex (ACC; r = 0.33, p = 0.27) and midbrain (r = 0.51, p = 0.08).
Discussion
PET imaging of the ECS has been extensively reviewed and, to our knowledge, this is the first study to investigate brain FAAH in humans with MDD [48,49,50]. No significant differences in [11C]CURB λk3 were observed between groups. This suggests that brain FAAH levels, as measured by [11C]CURB PET, do not differ between MDD and healthy controls. Greater apathy scores were associated with greater [11C]CURB binding in the mPFC, OFC, ACC, ventral striatum, and midbrain within the MDD group. These findings have important implications for FAAH in relation to apathy in MDD.
Contrary to our hypothesis, no significant differences in [11C]CURB λk3 were found between MDD participants and healthy controls across all brain ROIs. A lack of difference between groups could indicate no alterations in brain FAAH in MDD, or alternatively, if there is a subgroup with elevated [11C]CURB λk3 diluted by the heterogeneity of MDD, it is not prevalent enough to be detected in a group comparison. Were an elevation in FAAH present in the current study, it may have been expected that the presumed accompanying dysregulation of ECS could be reversed with FAAH inhibitors. Hence the negative findings of the present study could be considered consistent with the two negative clinical trials of FAAH inhibitors in MDEs [51, 52]. However, there are some limitations for this interpretation. One issue is that only one of the compounds, JNJ-42165279, has demonstrated high trough occupancy of approximately 80% during clinically relevant dosing in vivo in humans [53] whereas occupancy of SSR411298 is not known. In addition, there are limitations as to how the participant demographics of the clinical trials corresponded to the present study. The Phase II trial of SSR411298 sampled elderly patients with MDD [51] and, given the differences in the neurobiology and depressive symptom profiles between younger and older adults [54], the results of this clinical trial may not be generalizable. The other clinical trial using JNJ-42165279 in MDD with anxious distress [52] had no plan to selectively include patients with apathy, as the present study data was not available at the time of the study design, so the proportion of cases with apathy is not known.
The current study found a positive correlation between apathy and [11C]CURB λk3 in the PFC and ventral striatum. It could be speculated that elevated FAAH in the PFC and basal ganglia is detrimental to the function of the prefrontal-subcortical circuit, which may manifest as a diminution of motivation and goal-oriented behavior, thus leading to apathy. For example, AEA, a key FAAH substrate, may influence dopamine releasing neurons projecting from the substantial nigra to the dorsal putamen and from ventral tegmental area to the nucleus accumbens through altering release of glutamate and GABA neurotransmitters at these sites [55, 56]. Under such circumstances it is possible that a FAAH inhibitor might be therapeutic. Some caution to this perspective should be given, however, since there was no group difference between MDD and controls in the current study. Similarly, while FAAH has been shown to be elevated in animal models of anxiety in previous studies [57], the lack of correlation between [11C]CURB λk3 and anxiety in the MDD group may suggest that FAAH does not correspond to the anxiety associated with MDD in humans.
There are several limitations to the current study. First, due to the study’s cross-sectional design, we were unable to evaluate whether levels of brain FAAH change over time or under varying circumstances in our model. Second, [11C]CURB λk3 is an index of FAAH levels but also includes a component of non-displaceable binding; thus, in theory, changes in non-displaceable binding could influence the binding measure. However, approximately 90% of λk3 is directly attributable to specific binding [20], therefore it would take a massive effect of non-displaceable binding to substantially influence [11C]CURB λk3. A third limitation is that we did not pursue measurement of anhedonia, nor measurement of plasma anandamide, which could have been assessed in relation to λk3 and could be a direction for future study. Fourth, it is acknowledged that correlational analyses between indices of FAAH level and behavior may not capture complexities in the relationship between available FAAH levels and behavior, an issue exemplified in two relatively recent negative studies of FAAH inhibitors for PTSD [58, 59], despite beneficial effects on rodent fear extinction models. In addition, while several regions implicated in apathy circuitry had substantial correlations between λk3 and the MAES, the ACC did not. This would not preclude a potentially causal role for FAAH level on apathy in MDD as it is possible that changes in FAAH in some regional components of apathy circuitry are sufficient to influence apathy, particularly when other components of illness are present, a direction which could be evaluated further in preclinical models in the future. Finally, severity of anxiety was only modestly elevated in this sample of MDD participants so it is possible that correlations between anxiety and [11C]CURB λk3 could be present in a sample with greater anxiety.
The present study sought to investigate levels of brain FAAH in individuals with MDD compared to healthy controls using [11C]CURB PET imaging. We did not find any significant differences in [11C]CURB binding between the two groups in any of the brain regions examined, nor any relationship to anxiety. However, there was a significant positive association between [11C]CURB λk3 and apathy scores on the MAES. These findings have important implications for understanding the role of the ECS in apathy and motivation-related behavior. Future work may consider investigations of ECS in relation to apathy and shifting some focus of clinical trials of FAAH inhibitors in humans towards apathy.
Data availability
All data corresponding to the graphs presented during this study are included in this published article as supplementary files.
References
GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2022;9:137–50.
Vos T, Allen C, Arora M, Barber RM, Bhutta ZA, Brown A, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016;388:1545–602.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR* D report. Am J Psychiatry. 2006;163:1905–17.
Alnefeesi Y, Chen-Li D, Krane E, Jawad MY, Rodrigues NB, Ceban F, et al. Real-world effectiveness of ketamine in treatment-resistant depression: a systematic review & meta-analysis. J Psychiatr Res. 2022;151:693–709.
Alvares LDO, Genro BP, Diehl F, Quillfeldt JA. Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol Learn Mem. 2008;90:1–9.
Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol Ther. 2013;138:18–37.
Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–6.
Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–9.
Marco EM, Echeverry-Alzate V, López-Moreno JA, Giné E, Peñasco S, Viveros MP. Consequences of early life stress on the expression of endocannabinoid-related genes in the rat brain. Behav Pharmacol. 2014;25:547–56.
Van Gaal L, Pi-Sunyer X, Despres J-P, McCarthy C, Scheen A. Efficacy and safety of rimonabant for improvement of multiple cardiometabolic risk factors in overweight/obese patients: pooled 1-year data from the Rimonabant in Obesity (RIO) program. Diabetes Care. 2008;31:S229–S240.
Hungund BL, Vinod KY, Kassir SA, Basavarajappa BS, Yalamanchili R, Cooper TB, et al. Upregulation of CB1 receptors and agonist-stimulated [35S] GTPγS binding in the prefrontal cortex of depressed suicide victims. Mol Psychiatry. 2004;9:184–90.
Choi K, Le T, McGuire J, Xing G, Zhang L, Li H, et al. Expression pattern of the cannabinoid receptor genes in the frontal cortex of mood disorder patients and mice selectively bred for high and low fear. J Psychiatr Res. 2012;46:882–9.
McLaughlin RJ, Hill MN, Bambico FR, Stuhr KL, Gobbi G, Hillard CJ, et al. Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur Neuropsychopharmacol. 2012;22:664–71.
Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, et al. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex. 2008;18:1292–301.
Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–5.
Huang R, Zhang Y, Bai Y, Han B, Ju M, Chen B, et al. N6-Methyladenosine Modification of Fatty Acid Amide Hydrolase Messenger RNA in Circular RNA STAG1–Regulated Astrocyte Dysfunction and Depressive-like Behaviors. Biol Psychiatry. 2020;88:392–404.
Bambico FR, Cassano T, Dominguez-Lopez S, Katz N, Walker CD, Piomelli D, et al. Genetic deletion of fatty acid amide hydrolase alters emotional behavior and serotonergic transmission in the dorsal raphe, prefrontal cortex, and hippocampus. Neuropsychopharmacology. 2010;35:2083–2100.
Kirkedal C, Wegener G, Moreira F, Joca SRL, Liebenberg N. A dual inhibitor of FAAH and TRPV1 channels shows dose-dependent effect on depression-like behaviour in rats. Acta Neuropsychiatr. 2017;29:324–9.
Wilson AA, Garcia A, Parkes J, Houle S, Tong J, Vasdev N. 11C] CURB: Evaluation of a novel radiotracer for imaging fatty acid amide hydrolase by positron emission tomography. Nucl Med Biol. 2011;38:247–53.
Boileau I, Rusjan PM, Williams B, Mansouri E, Mizrahi R, Luca V, et al. Blocking of fatty acid amide hydrolase activity with PF-04457845 in human brain: a positron emission tomography study with the novel radioligand [11C] CURB. J Cereb Blood Flow Metab. 2015;35:1827–35.
Rusjan PM, Wilson AA, Mizrahi R, Boileau I, Chavez SE, Lobaugh NJ, et al. Mapping human brain fatty acid amide hydrolase activity with PET. J Cereb Blood Flow Metab. 2013;33:407–14.
Alexander JP, Cravatt BF. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem Biol. 2005;12:1179–87.
Boileau I, Tyndale RF, Williams B, Mansouri E, Westwood DJ, Foll B, et al. The fatty acid amide hydrolase C385A variant affects brain binding of the positron emission tomography tracer [11C] CURB. J Cereb Blood Flow Metab. 2015;35:1237–40.
Zimmermann T, Bartsch JC, Beer A, Lomazzo E, Guggenhuber S, Lange MD, et al. Impaired anandamide/palmitoylethanolamide signaling in hippocampal glutamatergic neurons alters synaptic plasticity, learning, and emotional responses. Neuropsychopharmacology. 2019;44:1377–88.
Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81.
Rafiei D, Kolla NJ. Elevated brain fatty acid amide hydrolase induces depressive-like phenotypes in rodent models: A review. Int J Mol Sci. 2021;22:1047.
Romero J, Hillard CJ, Calero M, Rabano A. Fatty acid amide hydrolase localization in the human central nervous system: an immunohistochemical study. Brain Res Mol Brain Res. 2002;100:85–93.
Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24.
Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161:1957–66.
Panagis G, Mackey B, Vlachou S. Cannabinoid regulation of brain reward processing with an emphasis on the role of CB1 receptors: a step back into the future. Front Psychiatry. 2014;5:92.
Covey DP, Hernandez E, Luján MÁ, Cheer JF. Chronic augmentation of endocannabinoid levels persistently increases dopaminergic encoding of reward cost and motivation. J Neurosci. 2021;41:6946–53.
Volkow ND, Hampson AJ, Baler RD. Don’t Worry, Be Happy: Endocannabinoids and cannabis at the intersection of stress and reward. Annu Rev Pharmacol Toxicol. 2017;57:285–308.
Schmidt ME, Liebowitz MR, Stein MB, Grunfeld J, Van Hove I, Simmons WK, et al. The effects of inhibition of fatty acid amide hydrolase (FAAH) by JNJ-42165279 in social anxiety disorder: a double-blind, randomized, placebo-controlled proof-of-concept study. Neuropsychopharmacology. 2021;46:1004–10.
Petrucci AS, LaFrance EM, Cuttler C. A comprehensive examination of the links between cannabis use and motivation. Subst Use Misuse. 2020;55:1155–64.
Lutz B, Marsicano G, Maldonado R, Hillard CJ. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci. 2015;16:705–18.
Silva HH, Tavares V, Neto BV, Cerqueira F, Medeiros R, Silva MG. FAAH rs324420 Polymorphism: biological pathways, impact on elite athletic performance and insights for sport medicine. Genes. 2023;14:1946.
Gaspersz R, Lamers F, Kent JM, Beekman AT, Smit JH, van Hemert AM, et al. Longitudinal predictive validity of the DSM-5 anxious distress specifier for clinical outcomes in a large cohort of patients with major depressive disorder. J Clin Psychiatry. 2017;78:207–13.
Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res. 1991;38:143–62.
Robert P, Lanctôt KL, Agüera-Ortiz L, Aalten P, Bremond F, Defrancesco M, et al. Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur Psychiatry. 2018;54:71–6.
Steffens DC, Fahed M, Manning KJ, Wang L. The neurobiology of apathy in depression and neurocognitive impairment in older adults: a review of epidemiological, clinical, neuropsychological and biological research. Transl Psychiatry. 2022;12:525.
Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121:553–8.
Levy ML, Cummings JL, Fairbanks LA, Masterman D, Miller BL, Craig AH, et al. Apathy is not depression. J Neuropsychiatry Clin Neurosci. 1998;10:314–9.
Husain M, Roiser JP. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat Rev Neurosci. 2018;19:470–84.
First M, Williams J, Karg R, Spitzer R. Structured Clinical Interview for DSM-5—Research Version. American Psychiatric Association: Arlington, VA; 2015.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56.
Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:5055.
Boileau I, Bloomfield PM, Rusjan P, Mizrahi R, Mufti A, Vitcu I, et al. Whole-body radiation dosimetry of 11C-carbonyl-URB694: a PET tracer for fatty acid amide hydrolase. J Nucl Med. 2014;55:1993–7.
Varlow C, Boileau I, Wey HY, Liang SH, Vasdev N. Classics in neuroimaging: imaging the endocannabinoid pathway with PET. ACS Chem Neurosci. 2020;11:1855–62.
Hou L, Rong J, Haider A, Ogasawara D, Varlow C, Schafroth MA, et al. Positron Emission Tomography Imaging of the Endocannabinoid System: Opportunities and Challenges in Radiotracer Development. J Med Chem. 2021;64:123–49.
Poluga C, Varlow C, Vasdev N, Boileau I, Best LM. Physiology of the endocannabinoid system: Imaging and the use of positron emission tomography (PET). In: Patel VB, Preedy VR, Martin CR, editors. Neurobiology and Physiology of the Endocannabinoid System. Burlington: Academic Press; 2023. p. 35–51.
Sanofi. An Eight-week Study of SSR411298 as Treatment for Major Depressive Disorder in Elderly Patients (FIDELIO). 2013. https://clinicaltrials.gov/study/NCT00822744.
Schmidt M, Simmons WK, Van Hove I, van der Ark P, Palmer J, Pemberton D, et al. A Phase 2a Randomized, Double-Blind, Placebo-Controlled Study Investigating the Efficacy and Safety of Adjunct Treatment With the FAAH Inhibitor JNJ-42165279 in Subjects With Major Depressive Disorder With Anxious Distress. ACNP 58th Annual Meet Orlando: Neuropsychopharmacol. 2019;44:78–229.
Postnov A, Schmidt ME, Pemberton DJ, de Hoon J, van Hecken A, van den Boer M, et al. Fatty Acid Amide Hydrolase Inhibition by JNJ‐42165279: a multiple‐ascending dose and a positron emission tomography study in healthy volunteers. Clin Transl Sci. 2018;11:397–404.
Hegeman AJM, Kok RM, Van Der Mast RC, Giltay EJ. Phenomenology of depression in older compared with younger adults: Meta-analysis. Br J Psychiatry. 2012;200:275–81.
Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang H-L, Zhang S, et al. Endocannabinoid actions on cortical terminals orchestrate local modulation of dopamine release in the nucleus accumbens. Neuron. 2017;96:1112–26.
Covey DP, Mateo Y, Sulzer D, Cheer JF, Lovinger DM. Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology. 2017;124:52–61.
Lee T, Hill MN, Lee FS. Developmental regulation of fear learning and anxiety behavior by endocannabinoids. Genes Brain Behav. 2016;15:108–24.
Mayo L. 5.4 The endocannabinoid as a novel therapeutic target in trauma-related disorders: A Randomized, controlled clinical trial and cross-section evidence from patients with trauma histories. acnp 63rd annual meeting: panels, mini-panels and study groups. Neuropsychopharmacology. 2024;49:1–64.
Pharmaceuticals J. A Multicenter Phase 2, 12-week Double-blind, Placebo-controlled, Randomized, Parallel-group Study of JZP150 for the Treatment of Posttraumatic Stress Disorder. NCT05178316. NLM. 2021-2023.
Funding
This work was funded by the Waypoint/University of Toronto Research Chair in Forensic Mental Health Science and a Canadian Institutes of Health Research (CIHR) Operating Grant, awarded to NJK; an Ontario Graduate Scholarship and a University of Toronto Open Fellowship Award, awarded to DR; and a CIHR Master’s Level Canada Graduate Scholarship, a Wilfred and Joyce Sex and Gender Fellowship, a University of Toronto Open Fellowship Award, awarded to MD. JHM is supported by a Canada Research Chair award.
Author information
Authors and Affiliations
Contributions
DR, MD, KD, JW, RA, LG, NJK, JHM: acquisition, analysis, and/or interpretation of the data. DR, MD, JHM, NJK, LG: preparation of the manuscript draft. JHM, IB, NJK: conception and/or design of the work. JHM, KD, RR, IB, JW, PR, NV, NJK: revised the manuscript critically for important intellectual content. DR, MD, JHM, KD, RR, IB, JW, PR, NV, RA, LG, NJK: final approval for it to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rafiei, D., De Pol, M., Meyer, J.H. et al. Fatty acid amide hydrolase in major depressive episodes: A [11C]CURB positron emission tomography study. Neuropsychopharmacol. (2025). https://doi.org/10.1038/s41386-025-02150-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41386-025-02150-y