Abstract
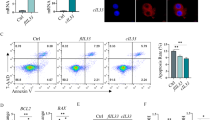
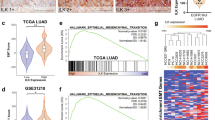
Dysregulations in protein kinases significantly contribute to the initiation, progression, and drug resistance in non-small cell lung cancer (NSCLC). Identification of novel oncogenic drivers within the human kinome is crucial for targeted therapy. In this study, we conducted a comprehensive analysis of the TCGA database and literature, pinpointing 16 candidate genes in lung cancer exhibiting frequent dysregulation and limited research. Our functional analysis revealed Serine/threonine kinase 31 (STK31) as a key player in driving tumor growth, in immune-competent mice, with minimal impact in nude mice. Further investigations unveiled upregulation of STK31 led to CD8+ T cell exhaustion. Mechanistically, STK31 induced CD8+ T cell exhaustion through the signal transducer and activator of transcription 3 (STAT3) - interleukin 6 (IL-6) signaling pathway. Direct interaction between STK31 and STAT3 activated the transcription of downstream oncogenic targets, such as IL-6, facilitating immune escape. Moreover, STK31 exhibited elevated expression levels in lung cancer tissues compared to adjacent tissues and displayed a significant correlation with poor prognosis in lung cancer patients. This study defines a critical role of STK31 in promoting immune escape through STAT3 activation, positioning it as a promising therapeutic target for lung cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The sequencing data of this study are available through GSA under accession number CRA016768. Other datasets during the study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, et al. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–89.
Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J Peking. 2021;134:783–91.
Osmani L, Askin F, Gabrielson E, Li QK. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): moving from targeted therapy to immunotherapy. Semin Cancer Biol. 2018;52:103–9.
Nong C, Guan P, Li L, Zhang H, Hu H. Tumor immunotherapy: mechanisms and clinical applications. MedComm Oncol. 2022;1:e8.
Konig D, Prince SS, Rothschild SI. Targeted therapy in advanced and metastatic non-small cell lung cancer. An update on treatment of the most important actionable oncogenic driver alterations. Cancers. 2021;13:804.
Cohen P. Protein kinases - the major drug targets of the twenty-first century? Nat Rev Drug Discov. 2002;1:309–15.
Roskoski R. A historical overview of protein kinases and their targeted small molecule inhibitors. Pharm Res. 2015;100:1–23.
Roskoski R. Properties of FDA-approved small molecule protein kinase inhibitors: a 2023 update. Pharm Res. 2023;187:106552.
Roskoski R. Futibatinib (Lytgobi) for cholangiocarcinoma. Trends Pharm Sci. 2023;44:190–1.
Attwood MM, Fabbro D, Sokolov AV, Knapp S, Schioth HB. Trends in kinase drug discovery: targets, indications and inhibitor design. Nat Rev Drug Discov. 2021;20:839–61.
Olesen C, Nyeng P, Kalisz M, Jensen TH, Moller M, Tommerup N, et al. Global gene expression analysis in fetal mouse ovaries with and without meiosis and comparison of selected genes with meiosis in the testis. Cell Tissue Res. 2007;328:207–21.
Bao J, Yuan S, Maestas A, Bhetwal BP, Schuster A, Yan W. Stk31 is dispensable for embryonic development and spermatogenesis in mice. Mol Reprod Dev. 2013;80:786.
Xiong J, Xing S, Dong Z, Niu L, Xu Q, Liu P, et al. STK31 regulates the proliferation and cell cycle of lung cancer cells via the Wnt/β‑catenin pathway and feedback regulation by c‑myc. Oncol Rep. 2020;43:395–404.
Bae DH, Kim HJ, Yoon BH, Park JL, Kim M, Kim SK, et al. STK31 upregulation is associated with chromatin remodeling in gastric cancer and induction of tumorigenicity in a xenograft mouse model. Oncol Rep. 2021;45:42.
Yokoe T, Tanaka F, Mimori K, Inoue H, Ohmachi T, Kusunoki M, et al. Efficient identification of a novel cancer/testis antigen for immunotherapy using three-step microarray analysis. Cancer Res. 2008;68:1074–82.
Gao H, Cai BB, Lu ZP, Wang GF, Gao Y, Miao Y, et al. Cancer-testis gene STK31 is regulated by methylation and promotes the development of pancreatic cancer. Cancer Med. 2023;12:7273–82.
Yang P, Meng M, Zhou QS. Oncogenic cancer/testis antigens are a hallmarker of cancer and a sensible target for cancer immunotherapy. BBA-Rev Cancer. 2021;1876:188558.
Fok KL, Chung CM, Yi SQ, Jiang X, Sun X, Chen H, et al. STK31 maintains the undifferentiated state of colon cancer cells. Carcinogenesis. 2012;33:2044–53.
Yu M, Peng Z, Qin M, Liu Y, Wang J, Zhang C, et al. Interferon-gamma induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol Cell. 2021;81:1216–30.e1219.
Mao Y, Sun S, Irvine KD. Role and regulation of Yap in KrasG12D-induced lung cancer. Oncotarget. 2017;8:110877–89.
Wang PJ, McCarrey JR, Yang F, Page DC. An abundance of X-linked genes expressed in spermatogonia. Nat Genet. 2001;27:422–6.
Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, et al. TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature. 2019;571:211–8.
Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, et al. TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature. 2019;571:265–9.
Li YJ, Zhang CY, Martincuks A, Herrmann A, Yu H. STAT proteins in cancer: orchestration of metabolism. Nat Rev Cancer. 2023;23:115–34.
Yeh HH, Lai WW, Chen HHW, Liu HS, Su WC. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene. 2006;25:4300–9.
Chang CH, Hsiao CF, Yeh YM, Chang GC, Tsai YH, Chen YM, et al. Circulating interleukin-6 level is a prognostic marker for survival in advanced nonsmall cell lung cancer patients treated with chemotherapy. Int J Cancer. 2013;132:1977–85.
Szyf M, Pakneshan P, Rabbani SA. DNA demethylation and cancer: therapeutic implications. Cancer Lett. 2004;211:133–43.
Meng H, Li GYA, Wei W, Bai YS, Feng Y, Fu M, et al. Epigenome-wide DNA methylation signature of benzo[a]pyrene exposure and their mediation roles in benzo[a]pyrene-associated lung cancer development. J Hazard Mater. 2021;416:125839.
Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–48.
Wang MW, Li SS, Guo WC, Wang LL, Huang JX, Zhuo JZ, et al. CHRAC1 promotes human lung cancer growth through regulating YAP transcriptional activity. Carcinogenesis. 2021;43:264–76.
Zhang H, Li S, Zhou R, Dong T, Zhang X, Yu M, et al. SRCAP complex promotes lung cancer progression by reprograming the oncogenic transcription of Hippo-YAP/TAZ signaling pathway. Cancer Lett. 2024;585:216667.
Burrack AL, Schmiechen ZC, Patterson MT, Miller EA, Spartz EJ, Rollins MR, et al. Distinct myeloid antigen-presenting cells dictate differential fates of tumor-specific CD8+ T cells in pancreatic cancer. JCI Insight. 2022;7:e151593.
Zhang HX, Zhang C, Tang H, Gao SS, Sun F, Yang Y, et al. CD2-associated protein contributes to hepatitis C, virus propagation and steatosis by disrupting insulin signaling. Hepatology. 2018;68:1710–25.
Acknowledgements
We thank Dr. Yu Xue in Huazhong University of Science and Technology for the bioinformatic analysis of cancer driver genes. This research was supported by NSFC National Natural Science Foundation and Ministry of Science and Technology of China (2023YFC2307304), This study also was supported by National Natural Science Foundation of China (No. 81902853) and in part by a Foundation of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (2022A07).
Author information
Authors and Affiliations
Contributions
SS contributed to experimental design, data analysis, and wrote the manuscript. WS contributed to experimental design, provided the patient samples and wrote the manuscript. LS designed experiments, analyzed data, performed experiments, data acquisition, and manuscript preparation. JL performed experiments, data acquisition, and manuscript preparation. SH performed experiments and data acquisition. LH provided the patient samples. MW provided the patient samples.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Lin, J., Huang, L. et al. STK31 drives tumor immune evasion through STAT3-IL-6 mediated CD8+ T cell exhaustion. Oncogene 44, 1452–1462 (2025). https://doi.org/10.1038/s41388-024-03271-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-03271-2