Abstract
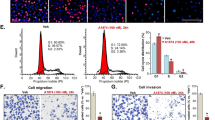
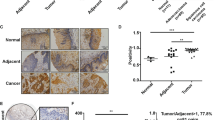
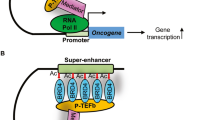
Tumor-associated macrophages (TAMs) in the tumor microenvironment play a key role in drug resistance, but the mechanisms underlying TAM polarization and its role in drug resistance remain unclear. Here, we identified BRD4 as a critical factor in TAM polarization and drug resistance in colorectal cancer (CRC). BRD4 deficiency in macrophages impaired M2-like TAM polarization, and tumors from myeloid-lineage specific Brd4 conditional knockout (Brd4-CKO) mice displayed a reduction in infiltrating M2-like TAMs and an enhanced anti-tumor microenvironment. Colon cancer cells treated with conditioned medium from polarized Brd4-deficient TAMs, as well as tumors in Brd4-CKO mice, were more sensitive to oxaliplatin. RNA-seq and cytokine microarray analysis revealed that mRNA and protein levels of PAI-1 were significantly decreased in Brd4-deficient polarized TAMs. BRD4 was recruited to the promoter of Serpine1, promoting SMAD-dependent PAI-1 expression. Supplementing Brd4-deficient TAMs with recombinant PAI-1 hampered the sensitivity of colon cancer cells to oxaliplatin. Moreover, PAI-1 inhibitor and oxaliplatin synergistically suppressed the growth of colon tumors. Clinically, the expression levels of BRD4 in TAMs and PAI-1 in tumors were elevated in CRC patients with chemoresistance, correlating with shorter recurrence-free survival. Collectively, our findings uncover a novel role for BRD4 in TAM polarization and drug resistance via PAI-1 upregulation, suggesting the BRD4/PAI-1 axis as a potential prognostic marker and therapeutic target in CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data are available from the corresponding author upon request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Johnson D, Chee CE, Wong W, Lam RCT, Tan IBH, Ma BBY. Current advances in targeted therapy for metastatic colorectal cancer - Clinical translation and future directions. Cancer Treat Rev. 2024;125:102700.
Sharma P, Goswami S, Raychaudhuri D, Siddiqui BA, Singh P, Nagarajan A, et al. Immune checkpoint therapy-current perspectives and future directions. Cell. 2023;186:1652–69.
Smith SM, Wachter K, Burris HA 3rd, Schilsky RL, George DJ, Peterson DE, et al. Clinical cancer advances 2021: ASCO’s report on progress against cancer. J Clin Oncol. 2021;39:1165–84.
Guo Y, Wang M, Zou Y, Jin L, Zhao Z, Liu Q, et al. Mechanisms of chemotherapeutic resistance and the application of targeted nanoparticles for enhanced chemotherapy in colorectal cancer. J Nanobiotechnol. 2022;20:371.
Bayik D, Lathia JD. Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer. 2021;21:526–36.
Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30:R921–R5.
Christofides A, Strauss L, Yeo A, Cao C, Charest A, Boussiotis VA. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23:1148–56.
Bleriot C, Dunsmore G, Alonso-Curbelo D, Ginhoux F. A temporal perspective for tumor-associated macrophage identities and functions. Cancer cell. 2024;42:747–58.
Pathria P, Louis TL, Varner JA. Targeting tumor-associated macrophages in cancer. Trends Immunol. 2019;40:310–27.
Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820.
Wang S, Wang J, Chen Z, Luo J, Guo W, Sun L, et al. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis Oncol. 2024;8:31.
Ha H, Oh EY, Lee HB. The role of plasminogen activator inhibitor 1 in renal and cardiovascular diseases. Nat Rev Nephrol. 2009;5:203–11.
Placencio VR, DeClerck YA. Plasminogen activator inhibitor-1 in cancer: rationale and insight for future therapeutic testing. Cancer Res. 2015;75:2969–74.
Rossi Sebastiano M, Pozzato C, Saliakoura M, Yang Z, Peng RW, Galie M, et al. ACSL3-PAI-1 signaling axis mediates tumor-stroma cross-talk promoting pancreatic cancer progression. Science advances. 2020;6:eabb9200.
Wang B, Gu B, Zhang T, Li X, Wang N, Ma C, et al. Good or bad: Paradox of plasminogen activator inhibitor 1 (PAI-1) in digestive system tumors. Cancer Lett. 2023;559:216117.
Dennler S, Itoh S, Vivien D, ten Dijke P, Huet S, Gauthier JM. Direct binding of Smad3 and Smad4 to critical TGF beta-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–100.
Datta PK, Blake MC, Moses HL. Regulation of plasminogen activator inhibitor-1 expression by transforming growth factor-beta -induced physical and functional interactions between smads and Sp1. J Biol Chem. 2000;275:40014–9.
Kubala MH, Punj V, Placencio-Hickok VR, Fang H, Fernandez GE, Sposto R, et al. Plasminogen activator inhibitor-1 promotes the recruitment and polarization of macrophages in cancer. Cell Rep. 2018;25:2177–91.e7.
Che Y, Wang J, Li Y, Lu Z, Huang J, Sun S, et al. Cisplatin-activated PAI-1 secretion in the cancer-associated fibroblasts with paracrine effects promoting esophageal squamous cell carcinoma progression and causing chemoresistance. Cell Death Dis. 2018;9:759.
Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282:13141–5.
Bao Y, Wu X, Chen J, Hu X, Zeng F, Cheng J, et al. Brd4 modulates the innate immune response through Mnk2-eIF4E pathway-dependent translational control of IkappaBalpha. Proc Natl Acad Sci USA. 2017;114:E3993–E4001.
Dong X, Hu X, Bao Y, Li G, Yang XD, Slauch JM, et al. Brd4 regulates NLRC4 inflammasome activation by facilitating IRF8-mediated transcription of Naips. J Cell Biol. 2021;220:e202005148.
Modi N, Chen Y, Dong X, Hu X, Lau GW, Wilson KT, et al. BRD4 regulates glycolysis-dependent Nos2 expression in macrophages upon H pylori infection. Cell Mol Gastroenterol Hepatol. 2024;17:292–308.e1.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63.
Fang X, Wu Y, Qin H, Zhao P, Shan M, Wang F, et al. Protocol for building an in vitro model of M2-like tumor-associated macrophages with lactic acid or conditioned medium from Lewis cells. STAR Protoc. 2024;5:103120.
Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Sig Transduct Target Ther. 2021;6:75.
Cassetta L, Pollard JW. A timeline of tumour-associated macrophage biology. Nat Rev Cancer. 2023;23:238–57.
Liu Y, Zhang Q, Xing B, Luo N, Gao R, Yu K, et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer cell. 2022;40:424–37.e5.
de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer cell. 2023;41:374–403.
Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol. 2022;15:61.
Vasan N, Baselga J, Hyman DM. A view on drug resistance in cancer. Nature. 2019;575:299–309.
Xie YH, Chen YX, Fang JY. Comprehensive review of targeted therapy for colorectal cancer. Sig Transduct Target Ther. 2020;5:22.
Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–20.
Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol cell. 1998;1:611–7.
Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharm. 2006;69:597–607.
Wrighton KH, Lin X, Feng XH. Phospho-control of TGF-beta superfamily signaling. Cell Res. 2009;19:8–20.
Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11:23–36.
Balsara RD, Ploplis VA. Plasminogen activator inhibitor-1: the double-edged sword in apoptosis. Thrombosis Haemost. 2008;100:1029–36.
Gorlatova NV, Cale JM, Elokdah H, Li D, Fan K, Warnock M, et al. Mechanism of inactivation of plasminogen activator inhibitor-1 by a small molecule inhibitor. J Biol Chem. 2007;282:9288–96.
Pelka K, Hofree M, Chen JH, Sarkizova S, Pirl JD, Jorgji V, et al. Spatially organized multicellular immune hubs in human colorectal cancer. Cell. 2021;184:4734–52.e20.
Mosser DM, Hamidzadeh K, Goncalves R. Macrophages and the maintenance of homeostasis. Cell Mol Immunol. 2021;18:579–87.
Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–44.
Park MD, Silvin A, Ginhoux F, Merad M. Macrophages in health and disease. Cell. 2022;185:4259–79.
Hu J, Li G, He X, Gao X, Pan D, Dong X, et al. Brd4 modulates metabolic endotoxemia-induced inflammation by regulating colonic macrophage infiltration in high-fat diet-fed mice. Commun Biol. 2024;7:1708.
Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPalpha immune checkpoint. Immunity. 2020;52:742–52.
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9.
Zorio E, Gilabert-Estelles J, Espana F, Ramon LA, Cosin R, Estelles A. Fibrinolysis: the key to new pathogenetic mechanisms. Curr Med Chem. 2008;15:923–9.
Bai X, Duan T, Shao J, Zhang Y, Xing G, Wang J, et al. CBX3 promotes multidrug resistance by suppressing ferroptosis in colorectal carcinoma via the CUL3/NRF2/GPX2 axis. Oncogene. 2025. https://doi.org/10.1038/s41388-025-03337-9.
Kalli M, Mpekris F, Charalambous A, Michael C, Stylianou C, Voutouri C, et al. Mechanical forces inducing oxaliplatin resistance in pancreatic cancer can be targeted by autophagy inhibition. Commun Biol. 2024;7:1581.
Schmierer B, Hill CS. TGFbeta-SMAD signal transduction: molecular specificity and functional flexibility. Nat Rev Mol Cell Biol. 2007;8:970–82.
Eren M, Painter CA, Gleaves LA, Schoenhard JA, Atkinson JB, Brown NJ, et al. Tissue- and agonist-specific regulation of human and murine plasminogen activator inhibitor-1 promoters in transgenic mice. J Thromb Haemost. 2003;1:2389–96.
Ross S, Cheung E, Petrakis TG, Howell M, Kraus WL, Hill CS. Smads orchestrate specific histone modifications and chromatin remodeling to activate transcription. EMBO J. 2006;25:4490–502.
Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku H, Fukamizu A, et al. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene. 2007;26:500–8.
Hu J, Pan D, Li G, Chen K, Hu X. Regulation of programmed cell death by Brd4. Cell Death Dis. 2022;13:1059.
Chung JY, Tang PC, Chan MK, Xue VW, Huang XR, Ng CS, et al. Smad3 is essential for polarization of tumor-associated neutrophils in non-small cell lung carcinoma. Nature Commun. 2023;14:1794.
Lei Z, Tang R, Wu Y, Mao C, Xue W, Shen J, et al. TGF-beta1 induces PD-1 expression in macrophages through SMAD3/STAT3 cooperative signaling in chronic inflammation. JCI insight. 2024;9:e165544.
Derynck R, Turley SJ, Akhurst RJ. TGFbeta biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18:9–34.
Donati B, Lorenzini E, Ciarrocchi A. BRD4 and Cancer: going beyond transcriptional regulation. Mol cancer. 2018;17:164.
Jung M, Gelato KA, Fernandez-Montalvan A, Siegel S, Haendler B. Targeting BET bromodomains for cancer treatment. Epigenomics. 2015;7:487–501.
Yin M, Guo Y, Hu R, Cai WL, Li Y, Pei S, et al. Potent BRD4 inhibitor suppresses cancer cell-macrophage interaction. Nat Commun. 2020;11:1833.
Wang H, Tang Y, Fang Y, Zhang M, Wang H, He Z, et al. Reprogramming tumor immune microenvironment (TIME) and metabolism via biomimetic targeting codelivery of shikonin/JQ1. Nano Lett. 2019;19:2935–44.
Bejarano L, Jordao MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–59.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68.
Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416.
Acknowledgements
We thank Cailing Yan from the Public Technology Service Center at Fujian Medical University for assistance with the IVIS® Spectrum In Vivo Imaging System analysis. This work was supported by the National Natural Science Foundation of China (81902842 to XMH and 81801974 to JFH), the Natural Science Foundation of Fujian Province (2022J01210 to DP, 2020J01615 to JFH and 2021J01669 to XMH), the Joint Funds for Innovation in Science and Technology, Fujian Province (2023Y9001 to XMH and 2020Y9006 to JFH), the Young and Middle-Aged Key Personnel Training Project of the Fujian Provincial Health Commission (2021GGA029 to DP); and the UIUC Research Board (RB24044) and CCIL Seed Grant to LFC.
Author information
Authors and Affiliations
Contributions
XMH and LFC designed the experiment; DP, JFH, GL, XMG, JW, LSJ, HL, YLC, YRZ and YHC performed the experiments; XMH, DP, JFH, GL, JW, XMG, LSJ, HL, YLC, YHC, YRZ, JJL, MZ, HC, and LFC analyzed the data; XMH, LFC and HC supervised the research; DP, XMH and LFC wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All human tissue investigations in this study were conducted in accordance with protocols approved by the Ethics Committee of the First Affiliated Hospital of Fujian Medical University (MRCTA, ECFAH of FMU 2021-423). Informed consent was obtained from all patients prior to the use of tumor tissues and clinical data. All animal experiments were approved by the Institutional Animal Care and Use Committee of Fujian Medical University (IACUC FJMU 2023-Y-0652).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, D., Hu, J., Li, G. et al. BRD4 regulates PAI-1 expression in tumor-associated macrophages to drive chemoresistance in colorectal cancer. Oncogene (2025). https://doi.org/10.1038/s41388-025-03453-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-025-03453-6