Abstract
Background
To determine the prevalence of pediatric Post-COVID-19 condition (PPCC), identify risk factors, and assess the quality of life in children with differing severities of acute COVID-19.
Methods
During a prospective longitudinal study with a 1-year follow-up, we compared non-hospitalized (mild) and hospitalized (severe) COVID-19 cases to a negatively tested control group.
Results
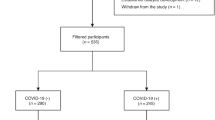
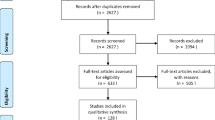
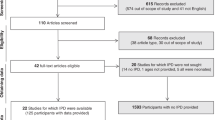
579 children were included in this study. Of these, 260 had mild acute disease (median age:8, IQR:6–10), 60 had severe acute disease (median age:1, IQR:0.1–4.0), and 259 tested negative for SARS-CoV-2 (NT) (median age:8, IQR:5-10).
At three months, 14.6% of the SARS-CoV-2 positive mild group (RR:6.31 (CI 95%: 2.71–14.67)) and 29.2% of the severe group (RR:12.95 (CI 95%: 5.37–31.23)) reported sequelae, versus 2.3% of the NT group. PPCC prevalence in the mild group decreased from 16.1% at one month to 4.4% at one year. Children with PPCC exhibited lower physical health-related quality of life scores and higher fatigue scores than the NT children.
Conclusions
Severe acute COVID-19 in children leads to a higher PPCC prevalence than in mild cases. PPCC prevalence decreases over time. Risk factors at three months include prior medical history, hospital admission, and persistent fatigue one month after a positive test.
Impact
-
We demonstrate children with severe COVID-19 are more likely to develop Post-COVID-19 condition than those with mild or no infections, and highlights the risk factors.
-
Here we have stratified by acute disease severity, prospectively included a negative control group, and have demonstrated the heterogeneity in prevalence when utilizing various recent definitions of post-COVID.
-
Identifying risk factors for pediatric post-COVID and highlighting the heterogeneity in prevalence based on various current definitions for post-COVID should aid in correctly identifying potential pediatric post-COVID cases, aiding in early intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
269,00 € per year
only 19,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
01 November 2024
In the original version of this article, the name of the author Anke H. Maitland-van der Zee was incorrectly structured. The author’s family name is ‘Maitland-van der Zee’.
References
Brodin, P. Immune responses to SARS-CoV-2 infection and vaccination in children. Semin Immunol. 69, 101794 (2023).
RIVM. Actuele informatie over het nieuwe coronavirus (COVID-19). Actuele informatie over het nieuwe coronavirus (COVID-19) | RIVM 1–1 https://www.rivm.nl/coronavirus-covid-19/actueel%0A. https://www.rivm.nl/nieuws/actuele-informatie-over-coronavirus (2020).
StatLine. Jongeren (0 tot 25 jaar); geslacht, leeftijd, migratieachtergrond, regio’s. StatLine: Jeugdmonitor https://jmopendata.cbs.nl/#/JM/nl/dataset/71009ned/table?dl=21563 (2023).
Wulf Hanson, S. et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA 328, 1604–1615 (2022).
Parisi, G. F. et al. Cross-sectional survey on long term sequelae of pediatric COVID-19 among Italian pediatricians. Children (Basel) 8, 769 (2021).
Osmanov, I. M. et al. Risk factors for long covid in previously hospitalised children using the ISARIC Global follow-up protocol: A prospective cohort study. Eur. Respir. J. 59, (2022).
Borch, L., Holm, M., Knudsen, M., Ellermann-Eriksen, S. & Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur. J. Pediatr. 181, 1597–1607 (2022).
Erol, N., Alpinar, A., Erol, C., Sari, E. & Alkan, K. Intriguing new faces of Covid-19: persisting clinical symptoms and cardiac effects in children. Cardiol. Young-. 32, 1085–1091 (2022).
Asadi-Pooya, A. A. et al. Long COVID in children and adolescents. World J. Pediatr. 17, 495–499 (2021).
Smane, L., Roge, I., Pucuka, Z. & Pavare, J. Clinical features of pediatric post-acute COVID-19: a descriptive retrospective follow-up study. Italian J. Pediatr. 47, 177 https://doi.org/10.1186/s13052-021-01127-z (2021).
Buonsenso, D. et al. Preliminary evidence on long COVID in children. Acta Paediatr. 110, 2208–2211 (2021).
Kikkenborg Berg, S. et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0-14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc. Health 6, 614–623 (2022).
Heching, H. J. et al. Electrocardiographic changes in non-hospitalised children with COVID-19. Cardiol. Young-. 32, 1910–1916 (2022).
Stephenson, T., Shafran, R. & Ladhani, S. N. Long COVID in children and adolescents. Curr. Opin. Infect. Dis. 35, 461–467 (2022).
Roge, I. et al. Comparison of Persistent Symptoms After COVID-19 and Other Non-SARS-CoV-2 Infections in Children. Front Pediatr. 9, 752385 (2021).
Zimmermann, P., Pittet, L. F. & Curtis, N. How Common is Long COVID in Children and Adolescents? Pediatr. Infect. Dis. J. 40, e482–e487 (2021).
Hahn, L. M. et al. Post–COVID-19 Condition in Children. JAMA Pediatr. 177, 1226–1228 (2023).
Bygdell, M., Kindblom, J. M., Martikainen, J., Li, H. & Nyberg, F. Incidence and Characteristics in Children with Post–COVID-19 Condition in Sweden. JAMA Netw. Open 6, e2324246 (2023).
Funk, A. L. et al. Post–COVID-19 Conditions Among Children 90 Days After SARS-CoV-2 Infection. JAMA Netw. Open 5, e2223253 (2022).
Brackel, C. L. H. et al. Pediatric long-COVID: An overlooked phenomenon? Pediatr. Pulmonol. 56, 2495–2502 (2021).
Izquierdo-Pujol, J. et al. Post COVID-19 Condition in Children and Adolescents: An Emerging Problem. Front Pediatr. 10, 894204 (2022).
Molteni, E. et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc. Health 5, 708–718 (2021).
Buonsenso, D. et al. Post-COVID Condition in Adults and Children Living in the Same Household in Italy: A Prospective Cohort Study Using the ISARIC Global Follow-Up Protocol. Front Pediatr. 10, 834875 (2022).
Gupta, M., Gupta, N. & Esang, M. Long COVID in children and adolescents. Prim Care Companion CNS Disord 24, 21r03218 (2022).
Pellegrino, R., Chiappini, E., Licari, A., Galli, L. & Marseglia, G. L. Prevalence and clinical presentation of long COVID in children: a systematic review. Eur. J. Pediatr. 181, 3995–4009 (2022).
Pierce, C. A. et al. COVID-19 and children. Science (1979) 377, 1144–1149 (2022).
Tulling, A. J. et al. Severe Pediatric COVID-19 and Multisystem Inflammatory Syndrome in Children From Wild-type to Population Immunity: A Prospective Multicenter Cohort Study With Real-time Reporting. Pediatr. Infect. Dis. J. https://doi.org/10.1097/INF.0000000000004098 (2023).
National Institute for Public Health and the Environment (RIVM). Varianten van het coronavirus SARS-CoV-2. https://www.rivm.nl/corona/actueel/virusvarianten.
National Institute for Health and Care Excellence, Practitioners, R. C. of G. & Scotland, H. I. COVID-19 rapid guideline: managing the long-term effects of COVID-19. NICE Guidelines 1–35 (2020).
Varni, J. W. The PedsQL Measurement Model. https://www.pedsql.org/ (2023).
van Muilekom, M. M. et al. Psychometric properties of the Patient-Reported Outcomes Measurement Information System (PROMIS®) Pediatric Anger Scale in the Dutch general population. Psychol. Assess. 33, 1261–1266 (2021).
Peersmann, S. H. M. et al. Psychometric properties and CAT performance of the PROMIS pediatric sleep disturbance, sleep-related impairment, and fatigue item banks in Dutch children and adolescents. Psychol. Assess. 34, 860–869 (2022).
PROMIS. https://www.healthmeasures.net/explore-measurement-systems/promis.
Klaufus, L. H. et al. Psychometric properties of the Dutch-Flemish PROMIS(®) pediatric item banks Anxiety and Depressive Symptoms in a general population. Qual. Life Res 30, 2683–2695 (2021).
Luijten, M. A. J., van Litsenburg, R. R. L., Terwee, C. B., Grootenhuis, M. A. & Haverman, L. Psychometric properties of the Patient-Reported Outcomes Measurement Information System (PROMIS®) pediatric item bank peer relationships in the Dutch general population. Qual. Life Res 30, 2061–2070 (2021).
Morello, R. et al. Risk factors for post-COVID-19 condition (Long Covid) in children: a prospective cohort study. EClinicalMedicine 59, 101961 (2023).
Di Sante, G. et al. Immunopathology of SARS-CoV-2 infection: a focus on t regulatory and b cell responses in children compared with adults. Children 9, 681 (2022).
Long COVID Kids. Feedback on the Long Covid Clinics. Preliminary healthcare experiences survey findings. https://www.longcovidkids.org/post/feedback-on-the-long-covid-clinics-preliminary-healthcare-experiences-survey-findings#viewer-dge2d (2023).
Buonsenso, D. et al. Paediatric long COVID studies should focus on clinical evaluations that examine the impact on daily life not just self-reported symptoms. Acta Paediatr. 113, 778–780 (2024).
Buonsenso, D. et al. Long-term outcomes of pediatric infections: from traditional infectious diseases to long Covid. Future Microbiol 17, 551–571 (2022).
Morrow, A. K. et al. Postacute/Long COVID in Pediatrics: Development of a Multidisciplinary Rehabilitation Clinic and Preliminary Case Series. Am. J. Phys. Med Rehabil. 100, 1140–1147 (2021).
NvK. NVK Handreiking Post-COVID Syndroom. https://www.nvk.nl/themas/kwaliteit/overige-kennisdocumenten/document?documentregistrationid=170917888 (2022).
A clinical case definition for post COVID-19 condition in children and adolescents by expert consensus, 16 February 2023. https://www.who.int/publications/i/item/WHO-2019-nCoV-Post-COVID-19-condition-CA-Clinical-case-definition-2023-1.
World Health Organization. a Clinical Case Definition for Post Covid-19 Condition in Children and Adolescents By Expert Consensus. 1–99 (2023).
Baldi, F. et al. Cardiopulmonary Exercise Testing in Children With Long COVID: A Case-controlled Study. Pediatr. Infect. Dis. J. (2024) https://doi.org/10.1097/INF.0000000000004371.
Delogu, A. B. et al. Autonomic cardiac function in children and adolescents with long COVID: a case-controlled study. Eur. J. Pediatr. 183, 2375–2382 (2024).
Buonsenso, D., Morello, R., De Rose, C., Spera, F. & Baldi, F. Long-term outcome of a child with postcovid condition: Role of cardiopulmonary exercise testing and 24-h Holter ECG to monitor treatment response and recovery. Pediatr. Pulmonol. 58, 2944–2946 (2023).
Spera, F. R. et al. Post-COVID Postural Orthostatic Tachycardia Syndrome and Inappropriate Sinus Tachycardia in the Pediatric Population. Curr. Clin. Microbiol Rep. 11, 115–125 (2024).
Davis, H. E., McCorkell, L., Vogel, J. M. & Topol, E. J. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21, 133–146 (2023).
Chen, E. Y., Morrow, A. K. & Malone, L. A. Exploring the Influence of Pre-Existing Conditions and Infection Factors on Pediatric Long COVID Symptoms and Quality of Life. Am. J. Phys. Med. Rehabil. (2023) https://doi.org/10.1097/PHM.0000000000002363.
Acknowledgements
For the collection of data for the PoCoCoChi study, we would like to acknowledge and thank the Public Health Service (GGD) Kennemerland. For the collection of the COPP Clinical data a large group of medical professionals and researchers (listed below) cooperated to make the data-collection possible. We Would like to acknowledge these as the ‘COPP study group’, the complete list of members can be found in the supplemental Digital Content Table E12. 1. Research grant for pediatric COVID research: ZonMw (10430072110007 and 10430102110009). 2. Research grant for pediatric COVID research: Bontius Stichting and the Leiden University Fund. 3. SAB (Stichting Steun Astma Bestrijding) Grant.
Author information
Authors and Affiliations
Contributions
Coen R. Lap and Caroline L.H. Brackel are responsible for the conception and design of the study, designed the data collection instruments, coordinated and supervised data collection, analyzed and interpreted the data, drafted, and critically reviewed and revised the manuscript for important intellectual content. Angelique M.A.M. Winkel, dr. Simone Hashimoto, Lieke C.E. Noij, Milly Haverkort, dr. Mattijs W. Alsem, Erik G.J. von Asmuth, dr. Michiel A.G.E. Bannier, dr Emmeline P. Buddingh, Adam J. Tulling, and dr. Gertjan Lugthart are responsible for the conception and design of the study, designed the data collection instruments, coordinated and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content. Dr. Johannes B. van Goudoever, dr Lotte Haverman, dr. Miriam G. Mooij, dr. Kim Oostrom, dr. Mariëlle W. Pijnenburg, and Sanne Kloosterman coordinated and supervised data collection, drafted, and critically reviewed and revised the manuscript for important intellectual content. Lorynn Teela and dr. Michiel Luijten designed the data collection instruments, coordinated and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content. dr. Anke H. Maitland – van der Zee, Dr. Debby Bogaert, dr. Giske Biesbroek, dr. Marlies A. van Houten, and dr. Suzanne W.J. Terheggen-Lagro are responsible for the conception and design of the study, designed the data collection instruments, coordinated and supervised data collection, and critically reviewed and revised the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Informed consent
Patient consent was required and obtained prior to inclusion in both the PoCoCoChi and COPP studies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lap, C.R., Brackel, C.L.H., Winkel, A.M.A.M. et al. Post-COVID-19 condition in children: epidemiological evidence stratified by acute disease severity. Pediatr Res 97, 1016–1024 (2025). https://doi.org/10.1038/s41390-024-03597-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-024-03597-3