Abstrct
Backgroud
Epidemiological data suggest that maternal occupational exposure to mixed phthalates comprising di(2-ethylhexyl) phthalate (DEHP) increases the risk of congenital heart disease (CHD). In this study, we used mice as an animal model to validate impact of first-trimester DEHP exposure on the risk of CHD in offspring, to elucidate the possible mechanisms and to provide a potential feasible intervention.
Methods and results
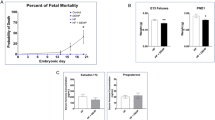
Eight-week-old C57BL/6J pregnant mice were randomly divided into standard and DEHP diet groups. The incidence of CHD in DEHP diet group offspring was up to 14.41% observed via Hematoxylin-eosin (HE) staining. Quantitative PCR analysis revealed that expression of key genes involved in cardiogenesis were suppressed at the transcriptional level, which may be due to decreased nuclear translocation of p65. The inhibition of DEHP on key genes was rescued to some extent by choline through driving p65 into nuclear. In the mice, supplementation of choline during DEHP exposure reduced the incidence of CHD in offspring from 14.41% to 4.63%.
Conclusions
Our study demonstrates that mice first-trimester DEHP exposure significantly increases the risk of CHD in the offspring via inhibiting mRNA levels of key genes in cardiogenesis, and choline could protect against the pathogenesis.
Impact
-
Our study provides key mechanistic insights into the risk of CHD by DEHP exposure during early pregnancy, and provides choline as a potentially effective intervention.
-
DEHP suppressed the expression of key genes involved in embryonic cardiac septum development at the transcriptional level via inhibiting nuclear translocation of p65.
-
Choline can play a role in rescuing the inhibition of DEHP on cardiogenesis genes via driving p65 translocate into the nuclear.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
269,00 € per year
only 19,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data presented in the paper are available from the corresponding author on reasonable request.
References
Liu, Y. et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J. Epidemiol. 48, 455–463 (2019).
Bruneau, B. G. The developmental genetics of congenital heart disease. Nature 451, 943–948 (2008).
Lage, K. et al. Genetic and environmental risk factors in congenital heart disease functionally converge in protein networks driving heart development. Proc. Natl Acad. Sci. USA 109, 14035–14040 (2012).
Zaidi, S. & Brueckner, M. Genetics and genomics of congenital heart disease. Circ. Res. 120, 923–940 (2017).
Wang, C. et al. Parental occupational exposures to endocrine disruptors and the risk of simple isolated congenital heart defects. Pediatr. Cardiol. 36, 1024–1037 (2015).
Sun, G. & Li, Y. Exposure to DBP induces the toxicity in early development and adverse effects on cardiac development in zebrafish (Danio rerio). Chemosphere 218, 76–82 (2019).
Tang, C. et al. The effect of maternal exposure to di-(2-ethylhexyl)-phthalate on fetal cardiac development in mice. J. Appl Toxicol. 38, 834–842 (2018).
Guo, Y. et al. Di-(2-ethylhexyl) phthalate exposure induces developmental toxicity in the mouse fetal heart via mitochondrial dysfunction. Cardiovasc. Toxicol. 25, 48–57 (2024).
Das, M. T., Ghosh, P. & Thakur, I. S. Intake estimates of phthalate esters for South Delhi population based on exposure media assessment. Environ. Pollut. 189, 118–125 (2014).
Tsatsakis, A. M. et al. Encyclopedia of Environmental Health, 2nd edn. (Elsevier, Oxford, 2019).
Zhao, H. et al. Investigation on metabolism of di(2-Ethylhexyl) phthalate in different trimesters of pregnant women. Environ. Sci. Technol. 52, 12851–12858 (2018).
Santos, S. et al. Maternal phthalate urine concentrations, fetal growth and adverse birth outcomes. A population-based prospective cohort study. Environ. Int. 151, 106443 (2021).
Ding, J., Xu, Z., Huang, W., Feng, L. & Yang, F. Organophosphate ester flame retardants and plasticizers in human placenta in Eastern China. Sci. Total Environ. 554–555, 211–217 (2016).
Tran, M. T. M. T. et al. Prenatal DEHP exposure predicts neurological disorders via transgenerational epigenetics. Sci. Rep. 13, 7399 (2023).
Oluwayiose, O. A. et al. Paternal preconception phthalate exposure alters sperm methylome and embryonic programming. Environ. Int 155, 106693 (2021).
Yu, D. et al. Prenatal di-(2-ethylhexyl) phthalate exposure induced myocardial cytotoxicity via the regulation of the NRG1-dependent ErbB2/ErbB4-PI3K/AKT signaling pathway in fetal mice. Ecotoxicol. Environ. Saf. 241, 113771 (2022).
Liu, Y. et al. An insight into sex-specific neurotoxicity and molecular mechanisms of DEHP: A critical review. Environ. Pollut. 316, 120673 (2023).
Sol, C. M. et al. Associations of maternal phthalate and bisphenol urine concentrations during pregnancy with childhood blood pressure in a population-based prospective cohort study. Environ. Int. 138, 105677 (2020).
Gao, H. et al. Combined effects of prenatal phthalate exposure on cardiometabolic risk score among 4- to 7-year-old children: MABC study. Chemosphere 311, 137135 (2023).
Tain, Y.-L., Hou, C.-Y., Chang-Chien, G.-P., Lin, S. & Hsu, C.-N. Resveratrol butyrate ester supplementation blunts the development of offspring hypertension in a maternal di-2-ethylhexyl phthalate exposure rat model. Nutrients 15, 697 (2023).
Wei, Z. et al. Maternal exposure to di-(2-ethylhexyl)phthalate alters kidney development through the renin-angiotensin system in offspring. Toxicol. Lett. 212, 212–221 (2012).
Huang, Y. et al. DEHP and DINP induce tissue- and gender-specific disturbances in fatty acid and lipidomic profiles in neonatal mice: a comparative study. Environ. Sci. Technol. 53, 12812–12822 (2019).
Posnack, N. G. et al. Exposure to phthalates affects calcium handling and intercellular connectivity of human stem cell-derived cardiomyocytes. PLoS ONE 10, e0121927 (2015).
Phthalates - ECHA. https://echa.europa.eu/hot-topics/phthalates (2010).
Blum, J. L., Chen, L.-C. & Zelikoff, J. T. Exposure to ambient particulate matter during specific gestational periods produces adverse obstetric consequences in mice. Environ. Health Perspect. 125, 077020 (2017).
Ravi, V., Jain, A., Taneja, A., Chatterjee, K. & Sundaresan, N. R. Isolation and culture of neonatal murine primary cardiomyocytes. Curr. Protoc. 1, e196 (2021).
Cai, K. et al. Nicotinamide mononucleotide alleviates cardiomyopathy phenotypes caused by short-chain enoyl-Coa hydratase 1 deficiency. JACC Basic Transl. Sci. 7, 348–362 (2022).
Rusyn, I., Peters, J. M. & Cunningham, M. L. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit. Rev. Toxicol. 36, 459–479 (2006).
Posnack, N. G., Swift, L. M., Kay, M. W., Lee, N. H. & Sarvazyan, N. Phthalate exposure changes the metabolic profile of cardiac muscle cells. Environ. Health Perspect. 120, 1243–1251 (2012).
Bustamante-Montes, L. P. et al. Phthalates exposure during pregnancy a study in a Mexican cohort. Toxicol. Rep. 8, 1040–1045 (2021).
Ye, T. et al. Effects of DEHP and its metabolite MEHP on cell apoptosis and the expression of steroidogenic genes in MLTC-1 cells. J. Environ. Hyg. 293, 281–287 (2018).
Pei, X., Duan, Z., Ma, M., Zhang, Y. & Guo, L. Role of Ca/CaN/NFAT signaling in IL-4 expression by splenic lymphocytes exposed to phthalate (2-ethylhexyl) ester in spleen lymphocytes. Mol. Biol. Rep. 41, 2129–2142 (2014).
Bushdid, P. B., Osinska, H., Waclaw, R. R., Molkentin, J. D. & Yutzey, K. E. NFATc3 and NFATc4 are required for cardiac development and mitochondrial function. Circ. Res. 92, 1305–1313 (2003).
Yang, Y. et al. Crosstalk between aryl hydrocarbon receptor and Wnt/β-catenin signaling pathway: Possible culprit of di (2-ethylhexyl) phthalate-mediated cardiotoxicity in zebrafish larvae. Sci. Total Environ. 907, 167907 (2024).
Sun, J. Exposure to Di-(2-ethylhexyl) phthalate drives ovarian dysfunction by inducing granulosa cell pyroptosis via the SLC39A5/NF-κB/NLRP3 axis. Ecotoxicol. Environ. Saf. 252, 114625 (2023).
Martínez-Razo, L. D., Martínez-Ibarra, A., Vázquez-Martínez, E. R. & Cerbón, M. The impact of di-(2-ethylhexyl) phthalate and mono(2-ethylhexyl) phthalate in placental development, function, and pathophysiology. Environ. Int. 146, 106228 (2021).
Karra, R., Knecht, A. K., Kikuchi, K. & Poss, K. D. Myocardial NF-κB activation is essential for zebrafish heart regeneration. Proc. Natl Acad. Sci. USA 112, 13255–13260 (2015).
Gaspar-Pereira, S. et al. The NF-κB subunit c-Rel stimulates cardiac hypertrophy and fibrosis. Am. J. Pathol. 180, 929–939 (2012).
Wu, K. et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 136, 501–515 (2020).
Li, X. et al. Distribution of phthalate metabolites between paired maternal–fetal samples. Environ. Sci. Technol. 52, 6626–6635 (2018).
Mose, T., Knudsen, L. E., Hedegaard, M. & Mortensen, G. K. Transplacental transfer of monomethyl phthalate and mono(2-ethylhexyl) phthalate in a human placenta perfusion system. Int. J. Toxicol. 26, 221–229 (2007).
Nappi, F. In-depth genomic analysis: The new challenge in congenital heart disease. Int J. Mol. Sci. 25, 1734 (2024).
Bruneau, B. G. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb. Perspect. Biol. 5, a008292 (2013).
Caudill, M. A. Pre- and postnatal health: evidence of increased choline needs. J. Am. Diet. Assoc. 110, 1198–1206 (2010).
Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (National Academies Press, Washington, 1998).
Funding
This research was funded by the grants from the National Natural Science Foundation of China (Nos. 82170305, 81770312) and grants from Discipline Leader Training Program of Children’s Hospital of Fudan University (No. EKXDPY202308) to F.W. This research was also funded by the grants from the National Natural Science Foundation of China (No. 82300428), Shanghai Sailing Program (No. 23YF1425500) to K.C. and the Open Program of NHC Key Laboratory of Neonatal Diseases (Fudan University).
Author information
Authors and Affiliations
Contributions
H.S., K.C. and F.W. contributed to the conception and design of the work. H.S., Z.Z. and A.S. contributed to the acquisition, analysis and interpretation of data. T.D., R.Z. and Y.S. contributed to the acquisition, analysis and interpretation of data as well as to supervision. H.S. drafted the manuscript. F.W., J.Z. and K.C. revised the manuscript for important intellectual content. All authors approved the final version of the manuscript and agreed to the version to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, H., Zhang, Z., Shen, A. et al. Maternal di(2-ethylhexyl) phthalate exposure increases the risk of congenital heart disease in mice offspring. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-03997-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-03997-z