Abstract
Background
The aim of this study was to evaluate the introduction of servo-controlled therapeutic hypothermia (TH) by a regional transport service and referring centres for infants with hypoxic ischaemic encephalopathy (HIE). The primary objective was to compare the time to reach 33–34 °C target temperature (TT).
Methods
This is a retrospective cohort study of neonatal transfers for TH across a large UK regional network from 2011 to 2021. Three cohorts were identified for comparison, defined by the setting of TH initiation: referring ‘Base’ centre or during ‘Transport’ and by the TH method: passive(Pass) or active(Act).
Results
A total of 315 infants were included. The TransportAct (n = 128) cohort achieved TT significantly earlier (280 min) than the TransportPass (n = 155) cohort (353 min, P < 0.001), with 84% vs 46% (OR 6.3, 95% Cl 3.3–11.8, P < 0.0001) achieving this within 6 h of birth. Introduction of BaseAct (n = 32) was associated with an additional 89 min reduction in time to TT (191 min, P < 0.0001), with more infants achieving this within 3 h (44% vs 19%; OR 3.3, 95% Cl 1.4–7.7, P < 0.01), and a shorter stabilisation time (110 vs 175 min, P < 0.001). Outcomes for infants were not different.
Conclusions
Compared with passive cooling, the introduction of transport and referring centre active TH improves temperature management of transferred infants with HIE, with more reaching therapeutic temperature within 6 h and fewer being overcooled.
Impact
-
Early therapeutic hypothermia (TH) improves survival without disability for infants with hypoxic ischaemic encephalopathy (HIE). However, many infants are born in centres without servo-controlled TH and rely on passive cooling prior to transfer.
-
This study demonstrates that the introduction of active TH by both the transport team and by referring centres is associated with significant improvements in time to reach target temperature, minimising overcooling and reducing transport stabilisation times.
-
Investment in active TH provision by all birth centres and transport teams could be a cost-effective method to reduce birth-related brain injury and improve outcomes of infants with HIE.
Similar content being viewed by others
Introduction
Hypoxic ischaemic encephalopathy (HIE) is a major cause of term brain injury globally with an incidence of 1–2/1000 live births.1 In the UK, approximately 500 infants per year with HIE require inter-hospital transfer for centralised intensive care with therapeutic hypothermia (TH).2 For infants with moderate/severe HIE, TH between 33 and 34 °C improves survival without disability when commenced within 6 h of birth, known as ‘the therapeutic window’.3,4
Active TH provided by a servo-controlled device is the optimal method for delivering TH5 compared to passive cooling,6 where there is a greater risk of overcooling (<33 °C) and potential harm.7,8 Servo-controlled TH is usually provided by neonatal intensive care units (NICUs), i.e. tertiary cooling centres, often resulting in passive cooling initially for infants with HIE born in non-cooling centres.
The safety and effectiveness of servo-controlled TH during neonatal transport9,10,11,12 has resulted in UK transport services adopting its use ahead of other countries.2,13,14 Infants with HIE born in non-cooling centres are at greater risk of poorer neurological outcomes compared to inborn infants.15,16 Globally, there is significant variability in the pathway of care for these infants, making it challenging for them to receive optimal treatment within 6 h.13,17,18 These disparities are a consequence of centralisation of neonatal care, regional variations in access to equipment, healthcare infrastructure, and geographical barriers.13,18,19
In the UK, the national HIE framework recommends that all levels of maternity/neonatal units should have access to servo-controlled TH equipment at birth, to enable earlier TH initiation.20 However, marked regional variation in the provision of active TH at birth exists.21 It is unclear if differences in the provision of active TH affect HIE therapeutic targets, transport metrics and short-term NICU outcomes for infants transferred for HIE. Understanding the impact of transport on infants requiring transfer for HIE and their thermoregulatory management were both identified as top ten neonatal transport research priorities.22
Our study aimed to understand how the implementation of servo-controlled TH by a regional transport service and adoption by referring centres affect important TH metrics. The primary objective was to compare the time to reach the 33–34 °C target temperature (TT). Secondary objectives explored the impact on transport process metrics and short-term infant outcomes.
Methods
Population
The Trent Perinatal and Central Neonatal Network (TPCNN) consists of 14 neonatal units; four NICUs (level 3) providing active TH, five Local Neonatal Units (level 2) and five Special Care Baby Units (level 1) (Supplementary File, Fig. 1). The CenTre Neonatal Transport Service provide transfer services across this network, undertaking 1298–1660 transfers per year, of which 2–3.3% are infants with HIE (Supplementary File, Table 1).
CenTre Neonatal Transport Service commenced use of servo-controlled TH devices in 2015; prior to this, infants were passively cooled in line with the procedure used in the TOBY trial.23 In 2016 and 2019, two Level 2 units switched from passive cooling to servo-controlled TH devices. The regional transport service and referring centres used the CritiCool servo-controlled TH device with the infant CureWrap (Belmont Medical Technologies, Billerica, Massachusetts). Changes in utilisation of dedicated crews and ambulances over the study period are outlined in Supplementary File, Table 2. Transfers of infants with HIE were considered as time-critical requiring team mobilisation within 60 min from base following referral.14,24
Infants referred for cooling transfer from April 2011 to March 2021 were identified from the routinely recorded transport database, and duplicated records were removed. All infants referred within TPCNN to the regional transport service for cooling transfer and completed transfer to a TPCNN cooling centre for ongoing management for HIE were included. The clinical decision to commence cooling was made by the referring centre and reviewed by the transport team on arrival. The referring centres and transport team followed a regional guideline to ensure that the infant met diagnostic criteria defined in the TOBY trial23 for initiating TH and for continuing TH treatment for HIE, warranting transfer to a regional cooling centre for ongoing care. The regional TH protocol includes that all infants ≥36 weeks gestation should be discussed for cooling transfer, and infants between 34 and 35 + 6 weeks gestation may be considered for cooling transfer at the discretion of the accepting tertiary cooling centre team.25
Infants not completing transfer, as cooling was no longer indicated after transport team assessment or the infant died before transfer, were excluded, along with infants referred from or completing transfer to a cooling centre outside of the TPCNN. Infants were split into one of three cohorts based on the combination of cooling method at their Base hospital (referring centre) and during Transport, with either passive(Pass) or active(Act) TH:
Cohort (1) BasePass/TransportPass (passive TH by both referring centre and transport);
Cohort (2) BasePass/TransportAct (passive TH by referring centre/active TH by transport);
Cohort (3) BaseAct/TransportAct (active TH by both referring centre and transport team).
Data collection
The change in access to active TH in the TPCNN resulted in this evaluation to understand the impact on the care pathway for infants with HIE. The service evaluation was registered with the University Hospitals of Leicester clinical audit department. The transport and tertiary team leads agreed to undertake infant data collection and co-developed the data collection proforma. Anonymised data were collected from routinely recorded clinical data in the transport database and local electronic record as detailed in Supplementary File, Table 3.
Infant characteristics included demographics, antenatal, intrapartum and resuscitation details. The primary objective was to establish the time to reach TT and achievement within 6h of age and TT at each stage of the transport pathway. Secondary objectives centred around transport team metrics including mobilisation and stabilisation times. Infant management and short-term outcomes on the NICU were also collected, definitions are outlined in the Supplementary File, Table 3. Standard magnetic resonance imaging (MRI) protocols were used consisting of T1, T2 and diffusion-weighted imaging (DWI) and reported locally by paediatric radiologists.
Statistical analysis
The final database was analysed using Stata SE V18 (StataCorp, College Station, Texas). Data returned as ‘unknown’ or incomplete were classified as ‘missing data’. Statistical significance was calculated using Chi-squared test and odds ratios (ORs) with 95% confidence intervals (Cls). For non-parametric, continuous data, a two-tailed Mann–Whitney test was used to compare medians. Statistical significance was defined as P < 0.05 and all data were unadjusted. Comparisons were made between cohorts 1 and 2 and between cohorts 2 and 3. Missing data were reported as a percentage of the total denominator for all cohorts combined.
Results
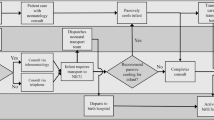
A total of 394 infants were referred from within the TPCNN of which 322 were included in the analysis (Fig. 1). Exclusions included 10 infants who did not complete transfer and 72 who were transferred outside of the TPCNN network.
Regional network refers to the infant referred within the Trent Perinatal and Central Neonatal Network. Three groups for comparison defined: (1) BasePass/TransportPass (passive therapeutic hypothermia (TH) by both the referring centre and Transport team), (2) BasePass/TransportAct (passive TH by referring centre and Active TH by transport team), (3) BaseAct/TransportAct (active TH by both referring centre and Transport team). *Infants did not meet the definitions of the final predefined groups for comparison.
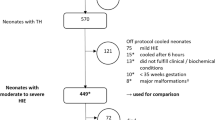
Of the 322 infants, the median age of referral to the transport service was 131 min (interquartile range (IQR) 88–190). Irrespective of the method of TH, 212 (67.7%) achieved TT within 6 h, and 245 (77.9%) arrived at the cooling centre within TT.
On admission to the cooling centre, 263 (81.9%) completed 72 h of TH. Of the 58 infants who did not complete full TH treatment, 22 died, 30 were considered to have mild HIE and 6 required significant cardiorespiratory support, leading to discontinuation of TH.
For the cohort analyses, seven infants were excluded as they did not fit into the defined groups, leaving 315 infants for comparison (Fig. 1 and Table 1). A total of 283 infants received BasePass prior to transport, of which 155 received TransportPass and 128 received TransportAct; 32 infants received both BaseAct and TransportAct. Six infants, two in each comparison group, were 34 + 0–35 + 6 weeks gestational age. The median transfer distances across comparison groups ranged between 34.4 and 52.5 km, with no difference observed between groups (Supplementary File, Table 4).
Temperature outcomes
Temperature on arrival of the transport team at referring BasePass centres for Cohorts 1 and 2 were similar with overall 31.3% (86/275) of babies in the TT range (Fig. 2). Compared to the BasePass infants, significantly more BaseAct infants (83.9%, 26/31) were in the TT range when the transport team arrived (OR 11.4, 95% Cl 4.0–32.7, P < 0.001).
Implementation of TransportAct resulted in significantly more infants departing the referring centre in the TT range with 95.3% (122/128) of infants vs 51.6% (80/155) with TransportPass (OR 19.1, 95% Cl 7.0–51.9, P < 0.0001). Fewer TransportAct infants departed overcooled at <33 °C (2.3%, 3/128) compared with TransportPass (14.2%, 22/155) (OR 0.15, 95% Cl 0.1-0.5, P < 0.001). On arrival at the receiving centre, 98.4% (124/126) of TransportAct infants were in TT on admission to the receiving cooling centre compared with only 57.2% (87/152) of TransportPass infants (OR 46.3, 95% Cl 9.0–237.9, P < 0.0001).
With the implementation of transport servo-controlled TH, the median age to TT significantly improved from 353 min (IQR 258–478) for BasePass/TransportPass to 280 min (IQR 210–341) for BasePass/TransportAct infants (P < 0.0001, Fig. 3), resulting in 84.4% (108/128) vs 46.3% (68/147) achieving TT within 6 h of age (Table 2). The introduction of BaseAct further reduced the age to TT by 89 min with a median age of 191 min (IQR 127–260, P < 0.001). Of the 32 infants who received BaseAct/TransportAct, 31 (96.9%) achieved TT within 6 h and were significantly more likely to achieve TT within 3 h of birth compared to infants receiving BasePass/TransportAct (Table 2).
Transport process metrics and infant outcomes
Following the introduction of active cooling for transport, teams were quicker mobilising and departing with a 43 min reduction in age to departing base (Table 2). TransportAct transfers had a median increase in stabilisation time of 30 min. Compared with TransportPass, TransportAct resulted in absolute 38.1% increase in the number of infants achieving TT within 6 h of age.
The introduction of BaseAct was associated with a significant increase in the median age of referral, with transport teams arriving at the referring centre at a later age, with no significant changes in median mobilisation and response times. Significantly more infants receiving BaseAct reached TT within 3 h of age and had a reduction in stabilisation time of 65 min compared with BasePass/TransportAct cohort, although fewer required respiratory support. There were no differences in important short-term NICU infant outcomes across the three cohorts (Table 3).
Discussion
Across a large regional network, the implementation of servo-controlled active TH by transport teams significantly improves the hypothermic management of infants with HIE, in particular the attainment of TT within 6 h of birth. When this is combined with active TH at the referring centre, there is an even greater improvement in hypothermic management. Importantly, at the point of transfer, there was almost a sevenfold reduction in the number of infants who were overcooled compared to those for whom only passive cooling was available. Overall, active TH at the referring centre and during transport was associated with earlier TH, leading to more infants reaching TT sooner and more than 95% starting the transport journey in the desired target range.
Delivering optimal treatment to infants with HIE born in non-cooling centres and requiring transfer is a global challenge, and understanding the impact of transport on these infants is a research priority.22 Servo-controlled TH devices are now used by all UK transport teams26 but use is more variable in other developed countries.13,14,18 Our study shows active TH during transport is associated with a significant reduction in the age to TT and a greater proportion achieving this within 6 h, bridging the gap for infants previously exposed to prolonged periods of suboptimal passive cooling6,12 who were required to await admission to a cooling centre for active TH.21 Despite this, we still observe a proportion of infants not reaching TT within 6 h of birth. Transport factors that may contribute to the delay achieving TT include age of referral and availability of a dedicated ambulance and team to enable faster mobilisation and timely arrival to the referring centre.27 These elements are important, along with the method of TH being delivered by transport teams. Our data suggest the use of active TH at birth centres may help mitigate some of these factors by reducing the time pressure on transport teams to arrive within the therapeutic window of 6 h, particularly in situations when transport teams may not be available to despatch immediately. Whilst we observed shorter stabilisation times in referral centres with active TH, we cannot say for certain that this relates to this change in practice or if other factors are also involved, as this cohort of infants required less invasive ventilation, which may have reduced turnaround times.
Delivery of active TH in referring hospitals may offer better neuroprotection by enabling earlier initiation of TH, with more infants reaching TT within 3 h of age. Earlier TH within 3 h of birth has been associated with greater neuroprotection, compared with later initiation (3–6 h), with animal studies reporting less neuronal loss,28,29,30 and observational human studies suggesting improved motor development and fewer seizures.31,32 Seizures in the context of HIE are associated with poorer outcomes.33,34 We did not observe significant differences in the number of infants with seizures or survival without seizures in infants receiving BaseAct, probably due to the small number of infants in this group.
The UK national HIE framework recommends all maternity/neonatal centres to have immediate access to servo-controlled TH.20 A recent study found 39% of UK births lacked this access at their birth centre and so relied on transport teams or cooling centres to initiate TH, with marked variation across regions.21 Gradual improvement in access has been observed, driven through quality improvement initiatives such as ‘Time=Brain’35,36; however, to standardise care for all infants requires further investment.
We show that access to TH equipment in both settings is an essential part of delivering efficient and optimal thermoregulatory care for infants transferred for HIE. This should be in parallel with education and training to support early identification and referral to transport teams to prompt discussion and guide decision-making. This approach is feasible in the UK, due to the neonatal healthcare and transport infrastructure2,37 and with the majority of transfers undertaken by road ambulance with a journey time under 2 h.17 For services covering larger geographical regions, with different transport modalities, or in settings with different healthcare infrastructure, limited neonatal expertise and resources at referring centres, this may be a less viable process.13,14,18
Strengths
This is the first large regional study of how stepwise implementation of active TH by transport and referring centres impacts achievement of HIE TH targets, thermoregulatory management and important transport metrics. Previous studies have either focused just on TH during transport or were small cohorts or single centred.10,11,38,39 This regional analysis of HIE management provides supportive evidence towards the recommendations outlined in the national HIE framework published after this study period.20
Limitations
The retrospective nature of the study limits our ability to control for data entry errors, incomplete records, missing data and potential reporting bias. To minimise these, we used standardised proformas and definitions and validated the returned data to check for inaccuracies.
Due to the small numbers within the comparison groups, our analysis did not adjust for potential confounders, and we did not observe any significant differences in short-term infant outcomes. A larger national study would be necessary to confirm any associations between improved thermal management and its impact on long-term neurodevelopmental outcomes and cost-effectiveness. Furthermore, a formal health economic evaluation and estimation of resource utilisation would be important to consider in future studies but is beyond the scope of this report.
The variability in MRI timing and reporting by different clinicians across centres could lead to inconsistencies in the reporting of scan findings.40,41 MRIs performed beyond day 7 of life can reduce the sensitivity of identifying hypoxic brain injury, especially on DWI and T2 scans.41,42 Paediatric radiologists reported the scans, but as a pragmatic service evaluation, we did not standardise the reports to any published scoring system.
Importantly, because of the retrospective nature of the study, we could not account for variations in clinical practice over time and across neonatal centres. We also could not assess whether the environmental exposures of ambulance transfer added additional stress to these high-risk infants or if being born in a non-cooling centre modifies the treatment effect of TH.
Conclusion
Implementation of active TH by transport and referring centres optimises the delivery of TH for infants treated for HIE who require transfer, most notably through earlier initiation and greater achievement of TT within 6 h of age. Investment in TH provision training and education to address disparities in TH care could potentially reduce brain injury and ease time pressures associated with HIE transfers for transport services. A larger national study validating our findings and associations with neurological outcomes could provide additional evidence for our study towards supporting national HIE recommendations.
Data availability
Anonymised transport data was provided from the CenTre Neonatal Transport database. All data are included in this manuscript or the Supplementary Material.
References
Shipley, L., Gale, C. & Sharkey, D. Trends in the incidence and management of hypoxic-ischaemic encephalopathy in the therapeutic hypothermia era: a national population study. Arch. Dis. Child. Fetal Neonatal Ed. 106, 529–534 (2021).
Leslie, A. et al. Tracking national neonatal transport activity and metrics using the UK Neonatal Transport Group dataset 2012–2021: a narrative review. Arch. Dis. Child. Fetal Neonatal Ed. 109, 460–466 (2024).
Azzopardi, D. et al. Implementation and conduct of therapeutic hypothermia for perinatal asphyxial encephalopathy in the UK-analysis of national data. PLoS ONE 7, e38504 (2012).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. CD003311 (2013).
National Institute for Health and Care Excellence. Therapeutic hypothermia with intracorporeal temperature monitoring for hypoxic perinatal brain injury. https://www.nice.org.uk/guidance/ipg347/resources/therapeutic-hypothermia-with-intracorporeal-temperature-monitoring-for-hypoxic-perinatal-brain-injury-pdf-1899867578267077 (2010).
Bourque, S. L. et al. A quality initiative for optimal therapeutic hypothermia during transport for neonates with neonatal encephalopathy. Pediatr. Qual. Saf. 3, e056 (2018).
Hallberg, B., Olson, L., Bartocci, M., Edqvist, I. & Blennow, M. Passive induction of hypothermia during transport of asphyxiated infants: a risk of excessive cooling. Acta Paediatr. 98, 942–946 (2009).
Eicher, D. J. et al. Moderate hypothermia in neonatal encephalopathy: efficacy outcomes. Pediatr. Neurol. 32, 11–17 (2005).
Sharma, A. Provision of therapeutic hypothermia in neonatal transport: a longitudinal study and review of literature. Cureus 7, e270 (2015).
Goel, N., Mohinuddin, S. M., Ratnavel, N., Kempley, S. & Sinha, A. Comparison of passive and servo-controlled active cooling for infants with hypoxic-ischemic encephalopathy during neonatal transfers. Am. J. Perinatol. 34, 19–25 (2017).
Chaudhary, R., Farrer, K., Broster, S., McRitchie, L. & Austin, T. Active versus passive cooling during neonatal transport. Pediatrics 132, 841 (2013).
Hagan, J. L. Meta-analysis comparing temperature on arrival at the referral hospital of newborns with hypoxic ischemic encephalopathy cooled with a servo-controlled device versus no device during transport. J. Neonatal Perinat. Med. 14, 29–41 (2021).
Lee, K. S. et al. Practice variations for therapeutic hypothermia in neonates with hypoxic-ischemic encephalopathy: an international survey. J. Pediatr. 274, 114181 (2024).
Lee, K.-S. Neonatal transport metrics and quality improvement in a regional transport service. Transl. Pediatr. 8, 233–245 (2019).
Shipley, L., Mistry, A. & Sharkey, D. Outcomes of neonatal hypoxic-ischaemic encephalopathy in centres with and without active therapeutic hypothermia: a nationwide propensity score-matched analysis. Arch. Dis. Child. Fetal Neonatal Ed. 107, 6–12 (2021).
Natarajan, G. et al. Effect of inborn vs. outborn delivery on neurodevelopmental outcomes in infants with hypoxic-ischemic encephalopathy: secondary analyses of the NICHD whole-body cooling trial. Pediatr. Res. 72, 414–419 (2012).
UK-NTG, Devon, C. & Jackson, A. UK Neonatal Transport Group Dataset. https://www.bapm.org/pages/ntg-dataset (2023).
Redpath, S. et al. Effectiveness of therapeutic hypothermia on transport within a large geographical area. Pediatrics 141, 723 (2018).
Fenton, A. C. & Leslie, A. The state of neonatal transport services in the UK. Arch. Dis. Child. Fetal Neonatal Ed. 97, F477–F481 (2012).
British Association of Perinatal Medicine. Therapeutic hypothermia for neonatal encephalopathy. A BAPM framework for practice. https://www.bapm.org/resources/237-therapeutic-hypothermia-for-neonatal-encephalopathy (2020).
Mistry, A., Shipley, L., Ojha, S. & Sharkey, D. Availability of active therapeutic hypothermia at birth for neonatal hypoxic ischaemic encephalopathy: a UK population study from 2011 to 2018. Arch. Dis. Child. Fetal Neonatal Ed. 507, 597–602 (2022).
Mistry, A., Leslie, A., Ojha, S. & Sharkey, D. Identifying neonatal transport research priorities: a modified Delphi consensus. Arch. Dis. Child. Fetal Neonatal Ed. 110, 43–50 (2024).
Azzopardi, D. V. et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N. Engl. J. Med. 361, 1349–1358 (2009).
British Association of Perinatal Medicine and Neonatal Transport Group. BAPM & NTG Neonatal Transport Dataset. https://www.bapm.org/pages/ntg-dataset (2016).
Jayasinghe, D. Management of Neonatal Encephalopathy (North Hub) East Midland Operational Delivery Network. https://nuhp.koha-ptfs.co.uk/cgi-bin/koha/opac-retrieve-file.pl?id=134a14e79919711248823abe68dfcb18 (2021).
Jackson, A. Neonatal Transport Group Dataset. https://www.bapm.org/pages/ntg-dataset (2019).
Ratnavel, N. Evaluating and improving neonatal transport services. Early Hum. Dev. 89, 851–853 (2013).
Gunn, A. J., Gunn, T. R., Gunning, M. I., Williams, C. E. & Gluckman, P. D. Neuroprotection with prolonged head cooling started before postischemic seizures in fetal sheep. Pediatrics 102, 1098–1106 (1998).
Roelfsema, V. et al. Window of opportunity of cerebral hypothermia for postischemic white matter injury in the near-term fetal sheep. J. Cereb. Blood Flow Metab. 24, 877–886 (2004).
Gunn, A. J. Cerebral hypothermia for prevention of brain injury following perinatal asphyxia. Curr. Opin. Pediatr. 12, 111–115 (2000).
Thoresen, M. et al. Time is brain: starting therapeutic hypothermia within three hours after birth improves motor outcome in asphyxiated newborns. Neonatology 104, 228–233 (2013).
Youn, Y.-A. et al. The hospital outcomes compared between the early and late hypothermia-treated groups in neonates. J. Matern. Fetal Neonatal Med. 29, 2288–2292 (2016).
Wirrell, E. C., Armstrong, E. A., Osman, L. D. & Yager, J. Y. Prolonged seizures exacerbate perinatal hypoxic-ischemic brain damage. Pediatr. Res. 50, 445–454 (2001).
Shah, D. K. et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Arch. Dis. Child Fetal Neonatal Ed. 99, F219–F224 (2014).
Reynolds, P. South East Coast Neonatal Network Time=Brain Quality Improvment initiative. https://www.networks.nhs.uk/nhs-networks/south-east-coast-neonatal-network/time-brain/time-brain (2025).
Mistry, A., Simpson, R. B., Ojha, S. & Sharkey D. Increasing availability of active therapeutic hypothermia for neonatal hypoxic ischaemic encephalopathy in the UK. Arch. Dis. Child Fetal Neonatal Ed. 110, 430–431 (2025).
British Association of Perinatal Medicine. Service and quality standards for provision of neonatal care in the UK. https://www.bapm.org/resources/service-and-quality-standards-for-provision-of-neonatal-care-in-the-uk (2022).
Stafford, T. D., Hagan, J. L., Sitler, C. G., Fernandes, C. J. & Kaiser, J. R. Therapeutic hypothermia during neonatal transport: active cooling helps reach the target. Ther. Hypothermia Temp. Manag. 7, 88–94 (2016).
Lumba, R., Mally, P., Espiritu, M. & Wachtel, E. V. Therapeutic hypothermia during neonatal transport at regional perinatal centers: active vs. passive cooling. J. Perinat. Med. 47, 365–369 (2019).
Austin, T. et al. Neonatal brain magnetic resonance imaging: clinical indication, acquisition and reporting. Arch. Dis. Child. Fetal Neonatal Ed. 109, 348–361 (2024).
Parmentier, C. E. J., de Vries, L. S. & Groenendaal, F. Magnetic resonance imaging in (near-)term infants with hypoxic-ischemic encephalopathy. Diagnostics 12, 645 (2022).
Bednarek, N. et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78, 1420–1427 (2012).
Acknowledgements
We would like to thank CenTre Neonatal Transport Service, who are hosted by the University Hospitals of Leicester NHS Trust and the East Midlands Operational Directive Network, for their support with this project.
Funding
A.M. was part of the project funded by the National Institute for Health Research (NIHR) i4i programme (II-LA-0715-20003), and D.S. was a co-investigator on the same award. D.S. is part-funded by the NIHR Children and Young People MedTech Cooperative. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
A.M., S.O. and D.S. conceptualised and formulated the study protocol. A.M. created the data collection proforma, and J.E., A. Lakshmanan, A.C., D.S. and S.O. provided input towards proforma development. A.M. led the implementation and conducted each stage of the study and the data analysis. N.I., J.F., D. Sobithadevi, D. Sham, B.J.B. and A. Lakshmanan performed local data collection. A. Leslie assisted in the interpretation of the data analysis. The first draft of the manuscript was written by A.M. and reviewed by all authors. All authors approved the final version for publication. D. S. is the guarantor of the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement and approval
Research Ethics Committee approval was not required as data was collected as part of a regional transport service evaluation, and no participants were recruited. The service evaluation was reviewed and registered at the University Hospitals of Leicester NHS Trust Clinical Audit department, Audit No: 12182.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mistry, A., Imolya, N., Fletcher, J. et al. Implementation of active therapeutic hypothermia across a regional transport network for infants transferred for neonatal encephalopathy. Pediatr Res (2025). https://doi.org/10.1038/s41390-025-04248-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41390-025-04248-x