Abstract
Study design
Review.
Objectives
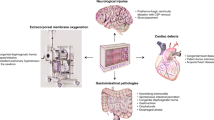
Clinical studies have shown that the hemodynamic management of patients following acute spinal cord injury (SCI) is an important aspect of their treatment for maintaining spinal cord (SC) perfusion and minimizing ischemic secondary injury to the SC. While this highlights the importance of ensuring adequate perfusion and oxygenation to the injured cord, a method for the real-time monitoring of these hemodynamic measures within the SC is lacking. The purpose of this review is to discuss current and potential methods for SC hemodynamic monitoring with special focus on applications using near-infrared spectroscopy (NIRS).
Methods
A literature search using the PubMed database. All peer-reviewed articles on NIRS monitoring of SC published from inception to May 2019 were reviewed.
Results
Among 125 papers related to SC hemodynamics monitoring, 26 focused on direct/indirect NIRS monitoring of the SC.
Discussion
Current options for continuous, non-invasive, and real-time monitoring of SC hemodynamics are challenging and limited in scope. As a relatively new technique, NIRS has been successfully used for monitoring human cerebral hemodynamics, and has shown promising results in intraoperative assessment of SC hemodynamics in both human and animal models. Although utilizing NIRS to monitor the SC has been validated, applying NIRS clinically following SCI requires further development and investigation.
Conclusions
NIRS is a promising non-invasive technique with the potential to provide real-time monitoring of relevant parameters in the SC. Currently, in its first developmental stages, further clinical and experimental studies are mandatory to ensure the validity and safety of NIRS techniques.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451–64.
Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte DW, Harrop JS, et al. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the surgical timing in acute spinal cord injury study (STASCIS). PLOS ONE. 2012;7:e32037.
Kong C, Hosseini A, Belanger L, Ronco J, Paquette S, Boyd M, et al. A prospective evaluation of hemodynamic management in acute spinal cord injury patients. Spinal Cord. 2013;51:466.
Hawryluk G, Whetstone W, Saigal R, Ferguson A, Talbott J, Bresnahan J, et al. Mean arterial blood pressure correlates with neurological recovery after human spinal cord injury: analysis of high frequency physiologic data. J Neurotrauma. 2015;32:1958–67.
Wing PC. Early acute management in adults with spinal cord injury: a clinical practice guideline for health-care providers. Who should read it? J spinal cord Med. 2008;31:360.
Ploumis A, Yadlapalli N, Fehlings M, Kwon B, Vaccaro A. A systematic review of the evidence supporting a role for vasopressor support in acute SCI. Spinal cord. 2010;48:356.
Casha S, Christie S. A systematic review of intensive cardiopulmonary management after spinal cord injury. J neurotrauma. 2011;28:1479–95.
Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB. et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. . 2001;24:254–64.
Cozzens JW, Prall JA, Holly LJN. The 2012 Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injury. Neurosurgery. 2013;72:2–3.
Acute spinal cord injury management: SurgicalCriticalCare.net; 2018 [Available from: http://www.surgicalcriticalcare.net/Guidelines/Acute%20Spinal%20Cord%20Injury%202018.pdf].
Inoue T, Manley GT, Patel N, Whetstone WD. Medical and surgical management after spinal cord injury: vasopressor usage, early surgerys, and complications. J Neurotrauma. 2014;31:284–91.
Werndle MC, Saadoun S, Phang I, Czosnyka M, Varsos GV, Czosnyka ZH, et al. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: initial findings of the injured spinal cord pressure evaluation study. Crit Care Med. 2014;42:646–55.
Phang I, Werndle MC, Saadoun S, Varsos G, Czosnyka M, Zoumprouli A, et al. Expansion duroplasty improves intraspinal pressure, spinal cord perfusion pressure, and vascular pressure reactivity index in patients with traumatic spinal cord injury: injured spinal cord pressure evaluation study. J Neurotrauma. 2015;32:865–74.
Phang I, Papadopoulos MC. Intraspinal pressure monitoring in a patient with spinal cord injury reveals different intradural compartments: Injured Spinal Cord Pressure Evaluation (ISCoPE) study. J Neurosurg. 2015;23:414–8.
Kwon BK, Curt A, Belanger LM, Bernardo A, Chan D, Markez JA, et al. Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: a prospective randomized trial. J Neurosurg: Spine. 2009;10:181–93.
Varsos GV, Werndle MC, Czosnyka ZH, Smielewski P, Kolias AG, Phang I, et al. Intraspinal pressure and spinal cord perfusion pressure after spinal cord injury: an observational study. J Neurosurg: Spine. 2015;23:763–71.
Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage . 2012;63:921–35.
Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–7.
Murkin J, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(suppl_1):i3–i13.
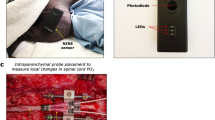
Macnab AJ, Gagnon RE, Gagnon FA. Near infrared spectroscopy for intraoperative monitoring of the spinal cord. Spine. 2002;27:17–20.
Hoshi Y. Functional near-infrared spectroscopy: current status and future prospects. J Biomed Opt. 2007;12:062106–9.
Madsen PL, Secher NH. Near-infrared oximetry of the brain. Prog Neurobiol. 1999;58:541–60.
Scholkmann F, Kleiser S, Metz AJ, Zimmermann R, Mata Pavia J, Wolf U, et al. A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. NeuroImage. 2014;85:6–27.
Ferrari M, Mottola L, Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can J Appl Physiol. 2004;29:463–87.
Cope M, Delpy D, Reynolds E, Wray S, Wyatt J, Van der Zee P. Methods of quantitating cerebral near infrared spectroscopy data. Oxyg Transp Tissue X: Springe. 1988;222:183–9.
Suehiro K, Funao T, Fujimoto Y, Mukai A, Nakamura M, Nishikawa K, et al. Transcutaneous near-infrared spectroscopy for monitoring spinal cord ischemia: an experimental study in swine. J Clin Monit. 2017;31:975–9.
Mesquita RC, D’Souza A, Bilfinger TV, Galler RM, Emanuel A, Schenkel SS, et al. Optical monitoring and detection of spinal cord ischemia. PLoS ONE. 2013;8:e83370.
de Haan P, Kalkman CJ. Spinal cord monitoring: Somatosensory- and motor-evoked potentials. Anesthesiol Clin North Am. 2001;19:923–45.
Macnab AJ, Gagnon RE, Gagnon FA, LeBlanc JG. NIRS monitoring of brain and spinal cord—detection of adverse intraoperative events. Spectroscopy . 2003;17:483–90.
Wallace MC, Tator CH. Spinal cord blood flow measured with microspheres following spinal cord injury in the rat. Can J Neurol Sci. 1986;13:91–6.
Çermik TF, Tuna F, Tuna H, Kaya M, Gültekin A, Yiğitbaşı ÖN, et al. Assessment of regional blood flow in cerebral motor and sensory areas in patients with spinal cord injury. Brain Res. 2006;1109:54–9.
Duhamel G, Callot V, Cozzone PJ, Kober F. Spinal cord blood flow measurement by arterial spin labeling. Magn Reson Med. 2008;59:846–54.
Duhamel G, Callot V, Decherchi P, Le Fur Y, Marqueste T, Cozzone PJ, et al. Mouse lumbar and cervical spinal cord blood flow measurements by arterial spin labeling: sensitivity optimization and first application. Magn Reson Med. 2009;62:430–9.
Morais DF, de Melo Neto JS, Meguins LC, Mussi SE, Ferraz Filho JRL, Tognola WA. Clinical applicability of magnetic resonance imaging in acute spinal cord trauma. Eur Spine J. 2014;23:1457–63.
Ellingson BM, Salamon N, Holly LT. Imaging techniques in spinal cord injury. World Neurosurg. 2014;82:1351–8.
Boezeman RP, Moll FL, Ünlü Ç, de Vries J-PP. Systematic review of clinical applications of monitoring muscle tissue oxygenation with near-infrared spectroscopy in vascular disease. Microvasc Res. 2016;104:11–22.
Nielsen HB. Systematic review of near-infrared spectroscopy determined cerebral oxygenation during non-cardiac surgery. Front Physiol. 2014;5:93.
Hamaoka T, McCully KK, Quaresima V, Yamamoto K, Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J Biomed Opt. 2007;12:062105.
Wolf EW. Dynamic detection of spinal cord position during postural changes using near‐infrared reflectometry. Neuromodulation: Technol Neural Interface. 2015;18:448–59.
Crameri RM, Cooper P, Sinclair PJ, Bryant G, Weston A. Effect of load during electrical stimulation training in spinal cord injury. Muscle Nerve. 2004;29:104–11.
Jan Y-K, Crane BA, Liao F, Woods JA, Ennis WJ. Comparison of muscle and skin perfusion over the ischial tuberosities in response to wheelchair tilt-in-space and recline angles in people with spinal cord injury. Arch Phys Med Rehabil. 2013;94:1990–6.
Ryan TE, Erickson ML, Young H-J, McCully KK. Case report: endurance electrical stimulation training improves skeletal muscle oxidative capacity in chronic spinal cord injury. Arch Phys Med Rehabil. 2013;94:2559–61.
Maikala RV, Bhambhani YN. Functional changes in cerebral and paraspinal muscle physiology of healthy women during exposure to whole-body vibration. Accident Analysis. Prevention . 2008;40:943–53.
Kawashima N, Nakazawa K, Akai M. Muscle oxygenation of the paralyzed lower limb in spinal cord-injured persons. Med Sci sports Exerc. 2005;37:915–21.
Muraki S, Fornusek C, Raymond J, Davis GM. Muscle oxygenation during prolonged electrical stimulation-evoked cycling in paraplegics. Appl Physiol, Nutr Metab. 2007;32:463–72.
Bhambhani Y, Tuchak C, Burnham R, Jeon J, Maikala R. Quadriceps muscle deoxygenation during functional electrical stimulation in adults with spinal cord injury. Spinal Cord. 2000;38:630.
Wang Y, Li Z, Lu C, Li J, Zhang L, Wang Y, (editors.) Hyperemia response of tissue oxygenation as assessed by the near infrared spectroscopy in persons with spinal cord injury. 3rd International Conference on Biomedical Engineering and Informatics; 2010: IEEE.
Li J, Li Z, Zhang M, Wang Y, Wang Y, Xin Q, et al. Wavelet analysis of sacral tissue oxygenation oscillations by near-infrared spectroscopy in persons with spinal cord injury. Microvasc Res. 2011;81:81–7.
Diaz D, Lafontant A, Neidrauer M, Weingarten MS, DiMaria-Ghalili RA, Scruggs E, et al. Pressure injury prediction using diffusely scattered light. J Biomed Opt. 2017;22:025003.
Draghici AE, Potart D, Hollmann JL, Pera V, Fang Q, DiMarzio CA, et al. Near infrared spectroscopy for measuring changes in bone hemoglobin content after exercise in individuals with spinal cord injury: NIRS for bone hemoglobin content. J Orthop Res. 2017;36:183–91.
Fornusek C, Davis GM. Cardiovascular and metabolic responses during functional electric stimulation cycling at different cadences. Arch Phys Med Rehabil. 2008;89:719–25.
Itoh M, Endo MY, Hojo T, Yoshimura M, Fukuoka Y. Characteristics of cardiovascular responses to an orthostatic challenge in trained spinal cord-injured individuals. J Physiol Anthropol. 2018;37:22.
Kunihara T, Shiiya N, Matsui Y, Yasuda K. Preliminary report of transesophageal monitoring of spinal cord ischemia using near-infrared spectrophotometry. J Cardiovasc Surg. 2004;45:95.
Kunihara T, Shiiya N, Matsuzaki K, Sata F, Matsui Y. Near-infrared spectrophotometry is useful to detect the beneficial pharmacological effects of alprostadil on spinal cord deoxygenation. Ann Thorac Cardiovasc Surg. 2008;14:376–81.
Radhakrishnan H, Senapati AK, Kashyap DR, Peng YB, Liu H. Light scattering from rat nervous system measured intraoperatively by near-infrared reflectance spectroscopy. J Biomed Opt. 2005;10:051405.
Liu H, Radhakrishnan H, Senapati AK, Hagains CE, Peswani D, Mathker A, et al. Near infrared and visible spectroscopic measurements to detect changes in light scattering and hemoglobin oxygen saturation from rat spinal cord during peripheral stimulation. Neuroimage . 2008;40:217–27.
LeMaire SA, Ochoa LN, Conklin LD, Widman RA, Clubb FJ Jr., Undar A, et al. Transcutaneous near-infrared spectroscopy for detection of regional spinal ischemia during intercostal artery ligation: Preliminary experimental results. J Thorac Cardiovasc Surg. 2006;132:1150–5.
Goguin A, Lesage F, Leblond H, Pelegrini-Issac M, Rossignol S, Benali H, et al. A low-cost implantable near-infrared imaging system of spinal cord activity in the cat. IEEE Trans Biomed circuits. 2010;4:329–35.
Tsiakaka O, Terosiet M, Romain O, Histace A, Benali H, Pradat P-F, et al., (editors.) In vivo NIRS monitoring in pig Spinal Cord tissues. Engineering in Medicine and Biology Society (EMBC), 37th Annual International Conference of the IEEE; 2015: IEEE.
Tsiakaka O, Feruglio S Toward the monitoring of the spinal cord: A feasibility study. Microelectronics. 2019;88:145–53.
Kogler AS, Bilfinger TV, Galler RM, Mesquita RC, Cutrone M, Schenkel SS, et al. Fiber-optic monitoring of spinal cord hemodynamics in experimental aortic occlusion. Anesthesiol: J Am Soc Anesthesiol. 2015;123:1362–73.
Busch DR, Davis J, Kogler A, Galler RM, Parthasarathy AB, Yodh AG, et al. Laser safety in fiber-optic monitoring of spinal cord hemodynamics: a preclinical evaluation. J Biomed Opt. 2018;23:065003. -.
von Aspern K, Haunschild J, Hoyer A, Luehr M, Bakhtiary F, Misfeld M, et al. Non-invasive spinal cord oxygenation monitoring: validating collateral network near-infrared spectroscopy for thoracoabdominal aortic aneurysm repair. Eur J Cardio-Thorac Surg. 2016;50:675–83.
Shadgan B, Kwon BK, Streijger F, Manouchehri N, So K, Shortt K, et al., (editors.) Optical monitoring of spinal cord hemodynamics, a feasibility study. Optical Diagnostics and Sensing XVII: Toward Point-of-CareDiagnostics; 2017: International Society for Optics and Photonics.
Shadgan B, Manouchehri N, So K, Shortt K, Fong A, Streijger F, et al., (editors.) Optical monitoring of spinal cord subcellular damage after acute spinal cord injury. Optical Diagnostics and Sensing XVIII: Toward Point-of-CareDiagnostics; 2018: International Society for Optics and Photonics.
Shadgan B, Macnab AJ, Fong A, Manouchehri N, So K, Shortt K, et al. Optical assessment of spinal cord tissue oxygenation using a miniaturized near infrared spectroscopy sensor. Journal of Neurotrauma. 2019. https://doi.org/10.1089/neu.2018.6208.
Boezeman RP, van Dongen EP, Morshuis WJ, Sonker U, Boezeman EH, Waanders FG, et al. Spinal near-infrared spectroscopy measurements during and after thoracoabdominal aortic aneurysm repair: a pilot study. Ann Thorac Surg. 2015;99:1267–74.
Etz CD, von Aspern K, Gudehus S, Luehr M, Girrbach FF, Ender J, et al. Near-infrared spectroscopy monitoring of the collateral network prior to, during, and after thoracoabdominal aortic repair: A pilot study. Eur J Vasc Endovasc Surg. 2013;46:651–6.
Berens RJ, Stuth EA, Robertson FA, Jaquiss RD, Hoffman GM, Troshynski TJ, et al. Near infrared spectroscopy monitoring during pediatric aortic coarctation repair. Pediatr Anesth. 2006;16:777–81.
Badner NH, Nicolaou G, Clarke CF, Forbes TL. Use of spinal near-infrared spectroscopy for monitoring spinal cord perfusion during endovascular thoracic aortic repairs. J Cardiothorac Vasc Anesth. 2011;25:316–9.
Moerman A, Van Herzeele I, Vanpeteghem C, Vermassen F, François K, Wouters P. Near-Infrared spectroscopy for monitoring spinal cord ischemia during hybrid thoracoabdominal aortic aneurysm repair. J Endovasc Ther. 2011;18:91–5.
Demir A, Erdemli Ö, Ünal U, Taşoğlu İ. Near-infrared spectroscopy monitoring of the spinal cord during type B aortic dissection surgery: NIRS monitoring in the spinal region. J Card Surg. 2013;28:291–4.
Luehr M, Mohr F-W, Etz C. Indirect neuromonitoring of the spinal cord by near-infrared spectroscopy of the paraspinous thoracic and lumbar muscles in aortic surgery. Thorac Cardiovasc Surg. 2016;64:333–5.
Amiri AR, Lee CH, Leung TS, Hetreed M, Craggs MD, Casey AT. Intraoperative assessment of human spinal cord perfusion using near infrared spectroscopy with indocyanine green tracer technique. Spine . 2013;13:1818–25.
Grus T, Lambert L, Rohn V, Klika T, Grusová G, Michálek P. Juxtarenal mycotic aneurysm as a complication of acute exacerbation of chronic cholecystitis treated by resection and replacement by a fresh allograft. Prague Med Report. 2016;117:54–60.
RBMD Griepp, EBMD. Griepp. Spinal Cord Perfusion and Protection During Descending Thoracic and Thoracoabdominal Aortic Surgery: The Collateral Network Concept. Ann Thorac Surg. 2007;83:S865–S9.
Meffert P, Bischoff MS, Brenner R, Siepe M, Beyersdorf F, Kari FA. Significance and function of different spinal collateral compartments following thoracic aortic surgery: immediate versus long-term flow compensation. Eur J Cardio-Thorac Surg. 2014;45:799–804.
Acknowledgements
BS holds a Scholar Award from the Michael Smith Foundation for Health Research. BKK is the Canada Research Chair in Spinal Cord Injury and the Dvorak Chair in Spine Trauma.
Author Contributions
TR was responsible for conducting the study and manuscript preparation. AM contributed to the study by analyzing the findings and drafting the manuscript. AC contributed to the study by article searching and manuscript preparation. AS contributed to the study by manuscript and graphic preparations. BK contributed the study by analyzing the findings, guiding the discussions, and reviewing the manuscript. BS was responsible for proposing and supervising the study and drafting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rashnavadi, T., Macnab, A., Cheung, A. et al. Monitoring spinal cord hemodynamics and tissue oxygenation: a review of the literature with special focus on the near-infrared spectroscopy technique. Spinal Cord 57, 617–625 (2019). https://doi.org/10.1038/s41393-019-0304-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-019-0304-2
This article is cited by
-
Non-invasive near-infrared spectroscopy assessment of the spinal neurovascular response in a patient with transverse myelitis: a case report
BMC Neurology (2022)
-
Spinal cord autoregulation using near-infrared spectroscopy under normal, hypovolemic, and post-fluid resuscitation conditions in a swine model: a comparison with cerebral autoregulation
Journal of Intensive Care (2020)
-
Transcutaneous contrast-enhanced ultrasound imaging of the posttraumatic spinal cord
Spinal Cord (2020)
-
Targeted Perfusion Therapy in Spinal Cord Trauma
Neurotherapeutics (2020)