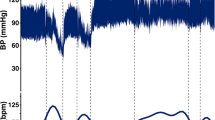
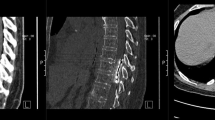
Abstract
Study design
Systematic review.
Objectives
Over the past decade, an increasing number of studies have demonstrated that epidural spinal cord stimulation (SCS) can successfully assist with neurorehabilitation following spinal cord injury (SCI). This approach is quickly garnering the attention of clinicians. Therefore, the potential benefits of individuals undergoing epidural SCS therapy to regain sensorimotor and autonomic control, must be considered along with the lessons learned from other studies on the risks associated with implantable systems.
Methods
Systematic analysis of literature, as well as preclinical and clinical reports.
Results
The use of SCS for neuropathic pain management has revealed that epidural electrodes can lose their therapeutic effects over time and lead to complications, such as electrode migration, infection, foreign body reactions, and even SCI. Several authors have also described the formation of a mass composed of glia, collagen, and fibrosis around epidural electrodes. Clinically, this mass can cause myelopathy and spinal compression, and it is only treatable by surgically removing both the electrode and scar tissue.
Conclusions
In order to reduce the risk of encapsulation, many innovative efforts focus on technological improvements of electrode biocompatibility; however, they require time and resources to develop and confirm safety and efficiency. Alternatively, some studies have demonstrated similar outcomes of non-invasive, transcutaneous SCS following SCI to those seen with epidural SCS, without the complications associated with implanted electrodes. Thus, transcutaneous SCS can be proposed as a promising candidate for a safer and more accessible SCS modality for some individuals with SCI.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Verrills P, Sinclair C, Barnard A. A review of spinal cord stimulation systems for chronic pain. J Pain Res. 2016;9:481.
Taccola G, Sayenko D, Gad P, Gerasimenko Y, Edgerton VR. And yet it moves: recovery of volitional control after spinal cord injury. Prog Neurobiol. 2018;160:64–81.
Weiss M, Mohr H. Spinal-cord stimulators help some patients, injure others. 2018. https://apnews.com/86ba45b0a4ad443fad1214622d13e6cb.
Sivanesan E, Bicket MC, Cohen SP. Retrospective analysis of complications associated with dorsal root ganglion stimulation for pain relief in the FDA MAUDE database. Regional Anesthesia Pain Med. 2019;44:100–6.
Taylor RS, Ryan J, O’Donnell R, Eldabe S, Kumar K, North RB. The cost-effectiveness of spinal cord stimulation in the treatment of failed back surgery syndrome. Clin J Pain. 2010;26:463–9.
Minassian K, McKay WB, Binder H, Hofstoetter US. Targeting lumbar spinal neural circuitry by epidural stimulation to restore motor function after spinal cord injury. Neurotherapeutics. 2016;13:284–94.
Rejc E, Angeli C, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS ONE. 2015;10:e0133998.
Grahn PJ, Lavrov IA, Sayenko DG, Van Straaten MG, Gill ML, Strommen JA, et al. Enabling task-specific volitional motor functions via spinal cord neuromodulation in a human with paraplegia. Mayo Clin Proc. 2017;92:544–54.
Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24:1677–82.
Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379:1244–50.
Wagner FB, Mignardot JB, Le Goff-Mignardot CG, Demesmaeker R, Komi S, Capogrosso M, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature. 2018;563:65–71.
Lu DC, Edgerton VR, Modaber M, AuYong N, Morikawa E, Zdunowski S, et al. Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Neurorehabil Neural Repair. 2016;30:951–62.
DiMarco AF, Geertman RT, Tabbaa K, Nemunaitis GA, Kowalski KE. Restoration of cough via spinal cord stimulation improves pulmonary function in tetraplegics. J Spinal Cord Med. 2019;1–7. https://doi.org/10.1080/10790268.2019.1699678. Online ahead of print.
Walter M, Lee AH, Kavanagh A, Phillips AA, Krassioukov AV. Epidural spinal cord stimulation acutely modulates lower urinary tract and bowel function following spinal cord injury: a case report. Front Physiol. 2018;9:1816.
Herrity AN, Williams CS, Angeli CA, Harkema SJ, Hubscher CH. Lumbosacral spinal cord epidural stimulation improves voiding function after human spinal cord injury. Sci Rep. 2018;8:8688.
Wernig A. No dawn yet of a new age in spinal cord rehabilitation. Brain. 2014;138:e362–e.
Wernig A, Müller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’is effective in spinal cord injured persons. Eur J Neurosci. 1995;7:823–9.
Wernig A, Muller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia. 1992;30:229–38.
Wernig A, Nanassy A, Muller S. Maintenance of locomotor abilities following Laufband (treadmill) therapy in para- and tetraplegic persons: follow-up studies. Spinal Cord. 1998;36:744–9.
Wernig A, Nanassy A, Müller S. Laufband (treadmill) therapy in incomplete paraplegia and tetraplegia. J Neurotrauma. 1999;16:719–26.
Dietz V, Wirz M, Colombo G, Curt A. Locomotor capacity and recovery of spinal cord function in paraplegic patients: a clinical and electrophysiological evaluation. Electroencephalogr Clin Neurophysiol. 1998;109:140–53.
Sherwood AM, Dimitrijevic MR, McKay WB. Evidence of subclinical brain influence in clinically complete spinal cord injury: discomplete SCI. J Neurol Sci. 1992;110:90–8.
McKay WB, Lim HK, Priebe MM, Stokic DS, Sherwood AM. Clinical neurophysiological assessment of residual motor control in post-spinal cord injury paralysis. Neurorehabil Neural Repair. 2004;18:144–53.
Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–409.
Darrow D, Balser D, Netoff TI, Krassioukov A, Phillips A, Parr A, et al. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma. 2019;36:2325–36.
Kleiber J-C, Marlier B, Bannwarth M, Theret E, Peruzzi P, Litre F. Is spinal cord stimulation safe? A review of 13 years of implantations and complications. Rev Neurologique. 2016;172:689–95.
Maldonado‐Naranjo AL, Frizon LA, Sabharwal NC, Xiao R, Hogue O, Lobel DA, et al. Rate of complications following spinal cord stimulation paddle electrode removal. Neuromodulation: technology at the Neural. Interface. 2018;21:513–9.
Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108:137–47.
Kumar K, Wilson JR, Taylor RS, Gupta S. Complications of spinal cord stimulation, suggestions to improve outcome, and financial impact. J Neurosurg Spine. 2006;5:191–203.
Pineda A. Dorsal column stimulation and its prospects. Surgical Neurol. 1975;4:157–63.
Pineda A. Complications of dorsal column stimulation. J Neurosurg. 1978;48:64–8.
Bendersky D, Yampolsky C. Is spinal cord stimulation safe? A review of its complications. World Neurosurg. 2014;82:1359–68.
Eldabe S, Buchser E, Duarte RV. Complications of spinal cord stimulation and peripheral nerve stimulation techniques: a review of the literature. Pain Med. 2016;17:325–36.
Shamji MF, Westwick HJ, Heary RF. Complications related to the use of spinal cord stimulation for managing persistent postoperative neuropathic pain after lumbar spinal surgery. Neurosurg Focus. 2015;39:E15.
Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg Spine. 2004;100:254–67.
Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain Pract. 2011;11:148–53.
Gazelka HM, Freeman ED, Hooten WM, Eldrige JS, Hoelzer BC, Mauck WD, et al. Incidence of clinically significant percutaneous spinal cord stimulator lead migration. Neuromodulation: technology at the Neural. Interface. 2015;18:123–5.
North RB, Lanning A, Hessels R, Cutchis PN. Spinal cord stimulation with percutaneous and plate electrodes: side effects and quantitative comparisons. Neurosurg Focus. 1997;2:E5.
Villavicencio AT, Leveque J-C, Rubin L, Bulsara K, Gorecki JP. Laminectomy versus percutaneous electrode placement for spinal cord stimulation. Neurosurgery. 2000;46:399–406.
Sandoe J, Barlow G, Chambers J, Gammage M, Guleri A, Howard P, et al. Report of a joint Working Party project on behalf of the British Society for Antimicrobial Chemotherapy (BSAC, host organization), British Heart Rhythm Society (BHRS), British Cardiovascular Society (BCS), British Heart Valve Society (BHVS) and British Society for Echocardiography (BSE). J Antimicrob Chemother. 2015;70:325–59.
Levy R, Henderson J, Slavin K, Simpson BA, Barolat G, Shipley J, et al. Incidence and avoidance of neurologic complications with paddle type spinal cord stimulation leads. Neuromodulation: technology at the neural. Interface. 2011;14:412–22.
Petraglia FW III, Farber SH, Gramer R, Verla T, Wang F, Thomas S, et al. The incidence of spinal cord injury in implantation of percutaneous and paddle electrodes for spinal cord stimulation. Neuromodulation: technology at the Neural. Interface. 2016;19:85–90.
Dam-Hieu P, Magro E, Seizeur R, Simon A, Quinio B. Cervical cord compression due to delayed scarring around epidural electrodes used in spinal cord stimulation: report of 2 cases. J Neurosurg Spine. 2010;12:409–12.
Hayek SM, Veizi E, Hanes M. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18:603–8.
Reynolds AF, Shetter AG. Scarring around cervical epidural stimulating electrode. Neurosurgery. 1983;13:63–5.
Deer T, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, et al. Neuromodulation appropriateness consensus committee: the appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:515–50.
Fransen P. Reversible late thoracic myelopathy and neurostimulation tolerance caused by fibrous scar tissue formation around the spinal cord stimulation electrode. Neuromodulation. 2015;18:759–61.
Lang P. The treatment of chronic pain by epidural spinal cord stimulation—a 15 year follow up; present status. Axone. 1997;18:71–3.
Henle C, Raab M, Cordeiro J, Doostkam S, Schulze-Bonhage A, Stieglitz T, et al. First long term in vivo study on subdurally implanted micro-ECoG electrodes, manufactured with a novel laser technology. Biomed Microdevices. 2011;13:59–68.
Wilk M, Hessler R, Mugridge K, Jolly C, Fehr M, Lenarz T, et al. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS ONE. 2016;11:e0147552.
Lin DP-Y, Chen JK-C, Tung T-H, Li LP-H. Differences in the impedance of cochlear implant devices within 24 hours of their implantation. PLoS ONE. 2019;14:e0222711.
Degenhart AD, Eles J, Dum R, Mischel JL, Smalianchuk I, Endler B, et al. Histological evaluation of a chronically-implanted electrocorticographic electrode grid in a non-human primate. J Neural Eng. 2016;13:046019.
Wissel K, Brandes G, Pütz N, Angrisani GL, Thieleke J, Lenarz T, et al. Platinum corrosion products from electrode contacts of human cochlear implants induce cell death in cell culture models. PLoS ONE. 2018;13:e0196649.
Schendel AA, Nonte MW, Vokoun C, Richner TJ, Brodnick SK, Atry F, et al. The effect of micro-ECoG substrate footprint on the meningeal tissue response. J Neural Eng. 2014;11:046011.
Wada E, Kawai H. Late onset cervical myelopathy secondary to fibrous scar tissue formation around the spinal cord stimulation electrode. Spinal Cord. 2010;48:646.
Cicuendez M, Munarriz PM, Castaño-Leon AM, Paredes I. Dorsal myelopathy secondary to epidural fibrous scar tissue around a spinal cord stimulation electrode: case report. J Neurosurg. 2012;17:598–601.
Al Tamimi M, Aoun SG, Gluf W. Spinal cord compression secondary to epidural fibrosis associated with percutaneously placed spinal cord stimulation electrodes: case report and review of the literature. World Neurosurg. 2017;104:1051. e1–e5.
Scranton RA, Skaribas IM, Simpson RK Jr. Spinal stimulator peri-electrode masses: case report. J Neurosurg Spine. 2015;22:70–4.
Nashold BS Jr, Friedman H. Dorsal column stimulation for control of pain. Preliminary report on 30 patients. J Neurosurg. 1972;36:590–7.
Krainick J-U, Thoden U, Riechert T. Pain reduction in amputees by long-term spinal cord stimulation: long-term follow-up study over 5 years. J Neurosurg. 1980;52:346–50.
Lennarson PJ, Guillen FT. Spinal cord compression from a foreign body reaction to spinal cord stimulation: a previously unreported complication. Spine. 2010;35:E1516–9.
Wloch A, Capelle HH, Saryyeva A, Krauss JK. Cervical myelopathy due to an epidural cervical mass after chronic cervical spinal cord stimulation. Stereotact Funct Neurosurg. 2013;91:265–9.
Guzzi G, Volpentesta G, Chirchiglia D, Della Torre A, Lavano F, Lavano A. Cervical spinal cord compression from delayed epidural scar tissue formation around plate lead for SCS. J Neurosurg Sci. 2019;63:337–43.
de Eulate-Beramendi SA, Santamarta-Liebana E, Leon RF, Saiz-Ayala A, Seijo-Fernandez FJ. Cervical cord compression secondary to epidural fibrous scar tissue around the spinal cord stimulation electrode. Neurology. 2016;64:1363–5.
Schendel AA, Thongpang S, Brodnick SK, Richner TJ, Lindevig BD, Krugner-Higby L, et al. A cranial window imaging method for monitoring vascular growth around chronically implanted micro-ECoG devices. J Neurosci Methods. 2013;218:121–30.
Sridar S, Churchward MA, Mushahwar VK, Todd KG, Elias AL. Peptide modification of polyimide-insulated microwires: Towards improved biocompatibility through reduced glial scarring. Acta Biomater. 2017;60:154–66.
Dimar JR II, Endriga DT, Carreon LY. Osteolysis and cervical cord compression secondary to silicone granuloma formation around a dorsal spinal cord stimulator: a case report. J Neurol Surg Rep. 2016;77:e67–e72.
Kim JE, Yang JH, Lee MK, Suh SW, Kang SW. Cervical myelopathy secondary to metallic irritation of the dura mater following insertion of a spinal cord stimulator in a patient with ossification of posterior longitudinal ligament. Pain Med. 2017;19:631–4.
Yamakawa T, Yamakawa T, Aou S, Ishizuka S, Suzuki M, Fujii M. Subdural electrode array manipulated by a shape memory alloy guidewire for minimally-invasive electrocorticogram recording. IEEE, World Automation Congress, Kobe, 2010. p. 1–6.
Yeager JD, Phillips DJ, Rector DM, Bahr DF. Characterization of flexible ECoG electrode arrays for chronic recording in awake rats. J Neurosci Methods. 2008;173:279–85.
Rubehn B, Bosman C, Oostenveld R, Fries P, Stieglitz T. A MEMS-based flexible multichannel ECoG-electrode array. J Neural Eng. 2009;6:036003.
Kim JJ, Gean AD. Imaging for the diagnosis and management of traumatic brain injury. Neurotherapeutics. 2011;8:39–53.
Norton L, Tegnell E, Toporek S, Reichert W. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials. 2005;26:3285–97.
Weaver CL, LaRosa JM, Luo X, Cui XT. Electrically controlled drug delivery from graphene oxide nanocomposite films. ACS Nano. 2014;8:1834–43.
Collier TO, Anderson JM, Brodbeck WG, Barber T, Healy KE. Inhibition of macrophage development and foreign body giant cell formation by hydrophilic interpenetrating polymer network. J Biomed Mater Res Part A. 2004;69:644–50.
Kolarcik CL, Bourbeau D, Azemi E, Rost E, Zhang L, Lagenaur CF, et al. In vivo effects of L1 coating on inflammation and neuronal health at the electrode–tissue interface in rat spinal cord and dorsal root ganglion. Acta Biomate. 2012;8:3561–75.
Vallejo‐Giraldo C, Krukiewicz K, Calaresu I, Zhu J, Palma M, Fernandez‐Yague M, et al. Attenuated glial reactivity on topographically functionalized poly (3, 4‐ethylenedioxythiophene): P‐toluene sulfonate (PEDOT: PTS) neuroelectrodes fabricated by microimprint lithography. Small. 2018;14:1800863.
Lavrov I, Dy CJ, Fong AJ, Gerasimenko Y, Courtine G, Zhong H, et al. Epidural stimulation induced modulation of spinal locomotor networks in adult spinal rats. J Neurosci. 2008;28:6022–9.
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–47.
Calvert JS, Manson GA, Grahn PJ, Sayenko DG. Preferential activation of spinal sensorimotor networks via lateralized transcutaneous spinal stimulation in neurologically intact humans. J Neurophysiol. 2019;122:2111–8.
Borgens RB, Shi R, Mohr TJ, Jaeger CB. Mammalian cortical astrocytes align themselves in a physiological voltage gradient. Exp Neurol. 1994;128:41–9.
Alexander JK, Fuss B, Colello RJ. Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol. 2006;2:93–103.
Pelletier SJ, Lagacé M, St-Amour I, Arsenault D, Cisbani G, Chabrat A, et al. The morphological and molecular changes of brain cells exposed to direct current electric field stimulation. Int J Neuropsychopharmacol. 2015;18:pyu090.
Metcalf MM, Shi R, Borgens RB. Endogenous ionic currents and voltages in amphibian embryos. J Exp Zool. 1994;268:307–22.
Taccola G, Gad P, Culaclii S, Ichiyama RM, Liu W, Edgerton VR. Using EMG to deliver lumbar dynamic electrical stimulation to facilitate cortico-spinal excitability. Brain Stimul. 2020;13:20–34.
Pancrazio JJ, Deku F, Ghazavi A, Stiller AM, Rihani R, Frewin CL, et al. Thinking small: progress on microscale neurostimulation technology. Neuromodulation: technology at the neural. Interface. 2017;20:745–52.
Lecomte A, Descamps E, Bergaud C. A review on mechanical considerations for chronically-implanted neural probes. J Neural Eng. 2018;15:031001.
Sohal HS, Jackson A, Jackson R, Clowry GJ, Vassilevski K, O’Neill A, et al. The sinusoidal probe: a new approach to improve electrode longevity. Front Neuroeng. 2014;7:10.
Du ZJ, Kolarcik CL, Kozai TD, Luebben SD, Sapp SA, Zheng XS, et al. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017;53:46–58.
Canales A, Park S, Kilias A, Anikeeva P. Multifunctional fibers as tools for neuroscience and neuroengineering. Acc Chem Res. 2018;51:829–38.
Garcia-Sandoval A, Pal A, Mishra AM, Sherman S, Parikh AR, Joshi-Imre A, et al. Chronic softening spinal cord stimulation arrays. J Neural Eng. 2018;15:045002.
Park SI, Brenner DS, Shin G, Morgan CD, Copits BA, Chung HU, et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat Biotechnol. 2015;33:1280.
Nguyen H, Arnob MMP, Becker AT, Wolfe JC, Hogan MK, Horner PJ, et al. Fabrication of multipoint side-firing optical fiber by laser micro-ablation. Opt Lett. 2017;42:1808–11.
Chang S-Y, Naganuma K, Kanazawa H, Sekino M, Onodera H, Kuniyoshi Y, editors. Applying Multichannel Optogenetic System for Epidural Spinal Cord Stimulation in Rats. 2018 40th Annual International Conference of the IEEE. EMBC. ThCT10.5, 2018.
Braun S, Ye Q, Radeloff A, Kiefer J, Gstoettner W, Tillein J. Protection of inner ear function after cochlear implantation: compound action potential measurements after local application of glucocorticoids in the guinea pig cochlea. ORL. 2011;73:219–28.
Van De Water TR, Dinh CT, Vivero R, Hoosien G, Eshraghi AA, Balkany TJ. Mechanisms of hearing loss from trauma and inflammation: otoprotective therapies from the laboratory to the clinic. Acta Oto-laryngologica. 2010;130:308–11.
Farah S, Doloff JC, Müller P, Sadraei A, Han HJ, Olafson K, et al. Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations. Nat Mater. 2019;18:892.
Hofstoetter US, Hofer C, Kern H, Danner SM, Mayr W, Dimitrijevic MR, et al. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed Tech Biomed Eng. 2013;58(Suppl 1):https://doi.org/10.1515/bmt-2013-4014.
Hofstoetter US, Krenn M, Danner SM, Hofer C, Kern H, McKay WB, et al. Augmentation of voluntary locomotor activity by transcutaneous spinal cord stimulation in motor-incomplete spinal cord-injured individuals. Artif Organs. 2015;39:E176–86.
Minassian K, Hofstoetter US, Danner SM, Mayr W, Bruce JA, McKay WB, et al. Spinal rhythm generation by step-induced feedback and transcutaneous posterior root stimulation in complete spinal cord-injured individuals. Neurorehabil Neural Repair. 2016;30:233–43.
Gerasimenko YP, Lu DC, Modaber M, Zdunowski S, Gad P, Sayenko DG, et al. Noninvasive reactivation of motor descending control after paralysis. J Neurotrauma. 2015;32:1968–80.
Gad P, Gerasimenko Y, Zdunowski S, Turner A, Sayenko D, Lu DC, et al. Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front Neurosci. 2017;11:333.
Inanici F, Samejima S, Gad P, Edgerton VR, Hofstetter CP, Moritz CT. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE engineering in medicine and biology. Society. 2018;26:1272–8.
Rath M, Vette AH, Ramasubramaniam S, Li K, Burdick J, Edgerton VR, et al. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma. 2018;35:2540–53.
Sayenko DG, Rath M, Ferguson AR, Burdick JW, Havton LA, Edgerton VR, et al. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J Neurotrauma. 2019;36:1435–50.
Gad PN, Kreydin E, Zhong H, Latack K, Edgerton VR. Non-invasive neuromodulation of spinal cord restores lower urinary tract function after paralysis. Front Neurosci. 2018;12:432.
Phillips AA, Squair JW, Sayenko DG, Edgerton VR, Gerasimenko Y, Krassioukov AV. An autonomic neuroprosthesis: noninvasive electrical spinal cord stimulation restores autonomic cardiovascular function in individuals with spinal cord injury. J Neurotrauma. 2018;35:446–51.
Hunter JP, Ashby P. Segmental effects of epidural spinal cord stimulation in humans. J Physiol. 1994;474:407–19.
Maertens de Noordhout A, Rothwell JC, Thompson PD, Day BL, Marsden CD. Percutaneous electrical stimulation of lumbosacral roots in man. J Neurol Neurosurg Psychiatry. 1988;51:174–81.
Minassian K, Persy I, Rattay F, Dimitrijevic MR, Hofer C, Kern H. Posterior root-muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve. 2007;35:327–36.
Rattay F, Minassian K, Dimitrijevic MR. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. quantitative analysis by computer modeling. Spinal Cord. 2000;38:473–89.
Ladenbauer J, Minassian K, Hofstoetter US, Dimitrijevic MR, Rattay F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: a computer simulation study. IEEE Trans Neural Syst Rehab. 2010;18:637–45.
Danner SM, Hofstoetter US, Ladenbauer J, Rattay F, Minassian K. Can the human lumbar posterior columns be stimulated by transcutaneous spinal cord stimulation? A modeling study. Artif Organs. 2011;35:257–62.
Jilge B, Minassian K, Rattay F, Dimitrijevic MR. Frequency-dependent selection of alternative spinal pathways with common periodic sensory input. Biol Cybern. 2004;91:359–76.
Minassian K, Persy I, Rattay F, Pinter MM, Kern H, Dimitrijevic MR. Human lumbar cord circuitries can be activated by extrinsic tonic input to generate locomotor-like activity. Hum Mov Sci. 2007;26:275–95.
Sayenko DG, Angeli C, Harkema SJ, Edgerton VR, Gerasimenko YP. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J Neurophysiol. 2014;111:1088–99.
Hofstoetter US, Freundl B, Binder H, Minassian K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: elicitation of posterior root-muscle reflexes. PLoS ONE. 2018;13:e0192013.
Minassian K, Hofstoetter US. Spinal cord stimulation and augmentative control strategies for leg movement after spinal paralysis in humans. CNS Neurosci Ther. 2016;22:262–70.
Willyard C. How a revolutionary technique got people with spinal-cord injuries back on their feet. Nature. 2019;572:20–5.
Hofstoetter US, McKay WB, Tansey KE, Mayr W, Kern H, Minassian K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J Spinal Cord Med. 2014;37:202–11.
Hofstoetter US, Freundl B, Danner SM, Krenn MJ, Mayr W, Binder H, et al. Transcutaneous spinal cord stimulation induces temporary attenuation of spasticity in individuals with spinal cord injury. J Neurotrauma. 2020;37:481–93.
Benfield J, Maknojia A, Epstein F. Progressive paraplegia from spinal cord stimulator lead fibrotic encapsulation: a case report. Am J Phys Med Rehab. 2016;95:e30–e3.
Aoun SG, El Ahmadieh T, Johnson ZD, Connors SW, Al Tamimi M. Reversible thoracic myelopathy after surgical decompression and removal of paddle neurostimulator lead and encasing fibrosis: technical video case report. Interdisciplinary. Neurosurgery. 2018;11:29–30.
Acknowledgements
GT is grateful to Dr. Elisa Ius for her excellent assistance in preparing the manuscript. The authors thank Dr. Gillian Hamilton and Rachel Markley for reviewing and proofreading the manuscript.
Funding
GT was supported by the Leonardo da Vinci 2019 fellowship from The Conference of Italian University Rectors and the Ministry of Education, University and Research (Italy). DS received philanthropic funding from Paula and Rusty Walter and Walter Oil & Gas Corp. The funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Author information
Authors and Affiliations
Contributions
GT conceptualized the review. GT, SB, PH, HCB, and DS screened potential studies. GT, SB, and DS performed the search. GT prepared the table. GT and DS interpreted results and drafted the manuscript. All authors revised the manuscript, and approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Taccola, G., Barber, S., Horner, P.J. et al. Complications of epidural spinal stimulation: lessons from the past and alternatives for the future. Spinal Cord 58, 1049–1059 (2020). https://doi.org/10.1038/s41393-020-0505-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-020-0505-8