Abstract
Opioid use disorders cause major morbidity and mortality, and there is a pressing need for novel mechanistic targets and biomarkers for diagnosis and prognosis. Exposure to mu-opioid receptor (MOR) agonists causes changes in cytokine and inflammatory protein networks in peripheral blood, and also in brain glia and neurons. Individuals with heroin use disorder (iHUD) show dysregulated levels of several cytokines in the blood. However, there is limited data on a comprehensive panel of such markers in iHUD versus healthy controls (HC), especially considered as a multi-target biomarker. We used a validated proximity extension assay for the relative quantification of 92 cytokines and inflammatory proteins in the serum of iHUD on medication-assisted therapy (MAT; n = 21), compared to HC (n = 24). Twenty-nine targets showed significant group differences (primarily iHUD>HC), surviving multiple comparison corrections (p = 0.05). These targets included 19 members of canonical cytokine families, including specific chemokines, interleukins, growth factors, and tumor necrosis factor (TNF)-related proteins. For dimensionality reduction, data from these 19 cytokines were entered into a principal component (PC) analysis, with PC1 scores showing significant group differences (iHUD > HC; p < 0.0001). A receiver-operating characteristic (ROC) curve analysis yielded an AUROC = 91.7% (p < 0.0001). This PC1 score remained a positive predictor of being in the HUD group in a multivariable logistic regression, that included select demographic/clinical variables. Overall, this study shows a panel of cytokines that differ significantly between iHUD and HC, providing a multi-target “cytokine biomarker score” for potential diagnostic purposes, and future examination of disease severity.
Similar content being viewed by others
Introduction
Heroin use disorders (HUD) and other opioid use disorders pose significant challenges to society, resulting in substantial morbidity and mortality [1], including a 2022 provisional estimate from the CDC of approximately 83,000 deaths attributed to opioid-related causes [2]. Heroin and other opioid compounds such as fentanyl act primarily as agonists at mu-opioid receptors (MOR), which mediate the direct effects as well as long-term pathophysiology of these compounds, both in the periphery and central nervous system. Although effective medication-assisted therapies (MAT) exist for HUD, such as oral maintenance with the MOR-agonist methadone or the partial MOR-agonist/kappa-opioid receptor (KOR) antagonist buprenorphine, a significant proportion of individuals with HUD (iHUD) discontinue treatment or relapse [3,4,5]. The underlying mechanisms for these undesirable outcomes remain unclear, highlighting an urgent need for novel mechanistically based treatments. There is also a pressing need for objective and quantitative biomarkers for HUD [6,7,8,9,10]. Due to their relative non-invasiveness and practicality, the measurement of blood-based biomarkers has emerged as a powerful approach in the study of diverse neuropsychiatric disorders [11, 12].
In addition to their actions on neuronal functions [13, 14], MOR agonists and their cognate receptors interact with complex networks of cytokines (e.g., chemokines, interleukins, growth factors, and tumor necrosis factor, TNF, -related proteins) [15, 16], signaling proteins that operate as interactively both in the periphery (e.g., in circulating leukocytes), and in central glia and neurons [17, 18]. Recent preclinical studies more broadly show that neuronal-glial interactions, often mediated by cytokines, are crucial in homeostatic functions including neuroplasticity and behavioral outcomes [18,19,20]. Importantly, circulating leukocytes and the cytokines they release may mediate some of the neurobiological and behavioral consequences of HUD [21, 22].
In support of this, recent preclinical studies in rodents have found that extended access fentanyl self-administration was correlated with blood levels of several cytokines (e.g., the chemokine CCL4, TNF-alpha, and interleukin-17) [23]. Intriguingly, this correlation was observed in a principal component analysis (PCA) factor that primarily contained translational indicators models of disease severity (e.g., escalation of drug intake, punished drug-taking, and progressive ratio behaviors) [23]. Furthermore, chronic i.v. oxycodone self-administration in mice caused upregulation in mRNA levels of several cytokine genes (or those for cognate receptors), such as oncostatin-M receptor, and several chemokines and TNF-related genes in the dorsal or ventral striatum [24]. Prior clinical studies have similarly detected differences in levels of some cytokines in iHUD compared to healthy controls (HC), with some discrepancies across studies, possibly due to methodological differences [25,26,27]. Given this translational gap, there is a clear need for analysis of a comprehensive panel of cytokines from major cytokine families, including chemokines, interleukins, growth factors, and TNF-related proteins, in the context of clinical HUD [7, 21].
The goals of this study were therefore twofold: First, to examine differences between iHUD and HC using a large and representative panel of cytokines and other inflammatory proteins [11, 28], aiming to identify novel targets that are potentially related to HUD [1, 29]. Secondly, to develop a multi-target (i.e., composite) “cytokine biomarker score” via dimensionality reduction with principal component analysis (PCA), and determine if it can robustly differentiate iHUD from HC, taking into account major demographic/clinical variables that may affect cytokine targets, especially age, body mass index (BMI), sleep, and perceived stress [30,31,32,33,34].
Methods
Participants and diagnostic procedures
Twenty-one iHUD and 24 age- and sex-matched HC were recruited for the current study. All iHUD were recruited from an inpatient drug addiction rehabilitation organization (Samaritan Daytop Village, NY). The HC were recruited from the surrounding communities, for matching purposes. This study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai, and all participants provided written informed consent. At the time of screening, all participants underwent a comprehensive clinical diagnostic interview, conducted by trained research staff under a clinical psychologist’s supervision, including the Addiction Severity Index (ASI) [35] and the M.I.N.I. neuropsychiatric interview [36] for DSM-5 diagnoses. On the day of the blood sample (mean = 113.7 days after screening; 95% CI:98.8–128.6), all participants also underwent a clinical interview to examine recent substance use status, providing a urine sample for drug testing, a carbon monoxide breath sample for nicotine use determination and salivary alcohol strip test. This interview also obtained basic vital signs (e.g., heart rate, temperature, blood pressure), and no abnormal findings were observed.
Inclusion criteria for all participants
Ability to understand and give informed consent in English, and 18–65 years of age. Inclusion criteria for iHUD specifically: Meet DSM-5 criteria for opioid use disorder, with heroin as the primary drug of choice or reason for treatment. We did not exclude iHUD with DSM-5 diagnosis of a drug use disorder other than opioid, as long as heroin was the primary drug of choice and reason for seeking treatment. All iHUD were inpatients in MAT and stabilized on methadone (n = 17) or buprenorphine (n = 4). Exclusion criteria for all participants: (1). DSM-5 diagnoses for psychotic disorders (e.g. schizophrenia) or neurodevelopmental disorders (e.g. autism). (2). History of head trauma with loss of consciousness (>30 min). (3). Neurological disease of central origin, including seizures. (4). Cardiovascular disease including high blood pressure. (5). Active infectious diseases such as hepatitis B/C or HIV/AIDS. (6). Other active medical conditions, including metabolic, endocrinological, oncological, or autoimmune diseases. The latter exclusions are based on the potential consequences of these conditions on neuropsychiatric and cytokine systems [37,38,39]. Exclusion criteria for HC specifically: Any current or prior diagnosis for any alcohol/substance use disorder.
Other medication use and comorbidities
In the iHUD, other than MAT, there was reported use of other common medications (e.g., of bupropion, selective serotonin reuptake inhibitors, quetiapine, and trazodone); the HC group reported instead primarily vitamin use (see Supplementary Table S1). None of the participants in either group had current exposure to corticosteroids, other major immunomodulatory or anti-inflammatory medications, or n-acetyl-cysteine, or opioids for the treatment of pain. As is common in persons in this diagnostic group, the iHUD also had some other lifetime substance use disorder diagnoses. Based on the MINI, of the total n = 21 iHUD, the following lifetime diagnoses were detected: n = 3 alcohol use disorder, n = 6 cocaine use disorder (n = 2 of these had both alcohol and cocaine use disorder); none with cannabis use disorder. We also analyzed years of regular use of alcohol and cannabis (based on the ASI), substances commonly used in the population overall.
Demographic, behavioral, and clinical variables
Sex, BMI, racial background, and age were examined as demographic variables. Hours of sleep in the night prior to the blood sample were also examined. Stress exposure, depression, and anxiety have been associated with changes in cytokine levels [11, 30, 40]. Therefore on the day of blood sampling, we examined perceived stress with the PSS-10 scale (score range: 0–40, denoting increasing perceived stress) [41, 42], self-rated anhedonia, dysphoria, pessimism, and fatigue with the Beck Depression Inventory (BDI-II; score range:0–63, scores ≤13 denote minimal depression) [43, 44], and somatic and cognitive symptoms of anxiety with the Beck Anxiety Inventory (BAI; score range: 0–63; scores ≤7 denote minimal anxiety) [45]. On the day of screening, the iHUD also completed the subjective opioid withdrawal scale (SOWS; score range 0–64, denoting increasing withdrawal) [46]. In the iHUD, we further examined methadone dose (documented report, or self-report if the former was unavailable) and duration of current abstinence from heroin, as well as age trajectory measures based on the ASI: age of first heroin use, the age of onset of regular use, and the number of years of regular use (excluding periods of abstinence) [47, 48]. A number of years of regular alcohol and cannabis use were also analyzed from the ASI (persons who did not endorse any regular use were assigned “0” for these values). A summary of demographic and clinical variables is in Table 1, and compared across groups with Mann–Whitney tests or Fisher’s exact tests.
Cytokine and inflammatory protein assay
A single blood sample was obtained by venipuncture, in the general time range of 09:00–17:00, at least one hour after the daily MAT dose for the iHUD. Samples were centrifuged (10 min at 1200 G) within ≈1 h, and serum was stored at −80 °C until the time of analysis. Serum samples were analyzed for relative levels of cytokines, using the validated Olink Target 96 Inflammation panel (Olink, Uppsala Sweden), following the manufacturer’s instructions [11], at the Human Immune Monitoring Center of the Icahn School of Medicine at Mount Sinai. This panel measures 92 different targets (principally chemokines, interleukins, growth factors, TNF-related molecules as well as other inflammation-related proteins; full target list: https://olink.com/products-services/target/inflammation/). The assay provides relative quantification of these targets, expressed as normalized protein units (NPX) on a log2 scale. Following established procedures, if a specific target in the panel yielded values for which ≥50% of the samples were lower than the limit of detection (LOD) within either the iHUD or HC group, the target overall was excluded from further analysis [11]. The remaining targets were analyzed, including individual values < LOD, as in previous studies [49]. Lastly, individual outliers (>±3 SD from the group mean), were removed from the individual target analyses. However, these outliers were later substituted by multiple imputation, for the principal component analysis (see below).
Statistical analyses for demographic and clinical variables
Sex and race distribution across groups was analyzed with Fisher’s exact test. Clinical data (e.g., age, BMI, sleep hours, PSS-10, BDI-II, BAI, and years of regular alcohol and cannabis use) were examined across groups with Mann–Whitney U tests.
Analyses of individual targets
Normalized protein units (NPX) for each target were analyzed with Wilcoxon’s rank-sum tests for group differences, and p-values were corrected for multiple comparisons, using the False Discovery Rate (FDR) approach (5% cutoff level) [50].
Principal component analysis based on 19 cytokines that differed significantly between iHUD and HC
For dimensionality reduction, after identifying the 29 targets that showed significant group differences (after correction for multiple comparisons), we focused on the subset thereof (19 targets) that are in canonical cytokine families. A principal component analysis (PCA) was carried out with these 19 canonical cytokines [11, 51], using centered and z-standardized individual values. Because PCA requires data for all relevant variables, outliers (greater than ±3 SD within each group mean) were replaced with a multiple imputation procedure (missMDA in R) [52]. Nineteen principal components were determined in the algorithm, with a 95% threshold for significance based on 1000 Monte Carlo simulations. Differences in principal component scores between iHUD and HC were examined non-parametrically (Mann–Whitney U test). A ROC curve was used to determine if the first principal component (PC1) score (that explained 40.9% variance) could be used as a diagnostic biomarker to separate iHUD and HC groups [53, 54]. As a follow-up, Spearman correlations were examined between these PC1 scores and demographic and clinical variables (as in Table 1), applying multiple comparison correction (FDR approach; 5% cutoff level).
Multiple logistic regression with group diagnosis (iHUD vs HC) as a binary outcome
The above PC1 scores were entered into a multiple logistic regression, together with age, BMI, sleep hours, PSS-10 (perceived stress) scores, and sex. The binary outcome was group diagnosis (HUD vs. HC). An ROC curve was also used to examine the performance of the multiple logistic regression in correctly classifying iHUD vs. HC.
Results
Demographics
Table 1 shows demographics and clinical variables. Age and hours slept in the night before blood sampling did not differ between groups. There were relatively more males than females in both iHUD and HC, but the contingency analysis was non-significant. There were relatively more persons of white race in the iHUD versus HC group. Also, iHUD had greater BMI, perceived stress scores (PSS-10), as well as depression (BDI-II), and anxiety (BAI) scores, compared to HC. Supplementary Fig. S1 shows that PSS-10 scores are widely distributed across participants, whereas BDI-II and BAI scores showed a robust “floor” effect (i.e., 0 scores), especially in the HC. The iHUD as a group reported very low SOWS (subjective withdrawal scores), at the time of screening. The mean duration of heroin abstinence in the iHUD was 198 days.
Cytokine and inflammatory target data
Comparison of iHUD versus HC
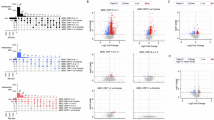
After excluding 14/92 targets due to ≥50% of the samples being <LOD (see “Methods” section), we compared the remaining 78 targets in iHUD vs. HC with Wilcoxon’s rank-sum tests. After FDR correction, 29 of these targets had significantly different NPX values between the two groups (Fig. 1A), of which 26 showed higher levels in iHUD vs. HC. Only 3 targets had the opposite profile (higher in HC vs. iHUD). Data summaries for these 29 targets are in Supplementary Table S2; targets that did not reach significance are in Supplementary Table S3. Fig. 1B shows the same data as Fig. 1A, as differences in mean scores (i.e., mean iHUD - mean HC), for visualization.
A Box and whisker plot for all the 29 targets that showed significant differences between iHUD and HC groups. Targets are organized by canonical cytokine families (19 targets; chemokines, interleukin-related, growth factors, and TNF-related), and the remainder as “other targets”. The box marks 25-75 percentiles; the midline marks the median and the whiskers mark 5-95 percentiles. p-values are shown for group comparisons per target, after FDR correction: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. B Re-plotting of panel A data as the difference in mean values in iHUD and HC (i.e., mean of iHUD - mean of HC). Positive values indicate that the target has levels iHUD>HC, and negative values indicate levels iHUD < HC. Full data for statistical comparisons are in the Supplementary Material.
Summary of 29 targets showing significant differences between the iHUD vs. HC
Of the 29 targets showing significant differences between the groups, 19 were members of canonical cytokine families. The cytokines with iHUD>HC levels were: the chemokines MCP1/CCL2, MCP3/CCL7, CCL19, and CXCL9, the interleukins IL6, IL10RB, IL15RA, IL18, OSM, and TRAIL, the growth factors CSF1, HGF, TGF-alpha and the TNF-related targets were TNFRSF9, TNFSF14, and CD40/TNFR5. By contrast, only three cytokines had the opposite profile, with HC > iHUD levels: CCL28, IL-7, and SCF. All the remaining 10 targets that reached group significance had an iHUD > HC profile. These targets were: eukaryotic translation initiation factor 4E [4E-BP1], adenosine deaminase [ADA], axin-1 [AXIN1], caspase-8 [CASP8], CD5, CD6, CUB ___domain-containing protein [CDCP1], extracellular newly identified receptor for advanced glycation end-products binding protein [EN.RAGE], signaling lymphocytic activation molecule 1 [SLAMF1], and signal-transducing adaptor molecule-binding protein [STAMBP] (marked “other targets” in Fig. 1).
Principal component analysis (PCA) on the 19 cytokines that differed significantly between iHUD vs. HC
Fig. 2 shows the PCA results based on the 19 cytokines that differed between the iHUD vs. HC (from Fig. 1; excluding the 10 significant “other targets” in the assay panel). The Scree plot in Fig. 2A shows that the first 2 principal components (i.e., PC1 and PC2) accounted for 40.9% and 15.8% of variance, respectively (further PCs accounted for relatively small proportions of variance). As shown in Fig. 2B, C, PC1 scores were significantly greater in the iHUD vs. HC (Mann–Whitney U = 36; p < 0.0001), whereas PC2 scores did not differ significantly between groups (not shown). An ROC curve of the PC1 scores in Fig. 2D, with iHUD and HC as the binary outcomes, shows a univariate AUROC = 91.7 (p < 0.0001). Loadings (Eigenvector*√Eigenvalue) for the 19 cytokines in PC1 are shown in the Supplementary Material (Table S4), to illustrate the contribution of individual cytokines to the overall PC score. As expected, the sign of PC loadings differed between the 16 cytokines that had iHUD>HC values versus the 3 cytokines that had HC > iHUD values (i.e., CCL28, IL-7, and SCF) (Supplementary Table S4).
A Scree plot, showing the proportion of variance accounted for by consecutive principal components (PC). B Scatter plot for individual scores for PC1 and PC2. C Comparison of PC1 scores between iHUD (n = 21) and HC (n = 24). D Receiver-operating characteristic (ROC) curve for PC1 scores as a measure to detect HUD vs HC as binary diagnostic outcomes.
Multiple logistic regression for HUD and HC as binary diagnostic outcomes, examining cytokine PC1 scores and major demographic and clinical variables
We carried out a multiple logistic regression with group membership as the outcome (iHUD vs. HC), with the following variables: cytokine PC1 scores (from Fig. 2), sex, age, BMI, sleep hours, and perceived stress (PSS-10) scores. Depression and anxiety scores (BDI-II and BAI instruments, respectively) were not entered in the regression due to “floor” effects in HC (Supplementary Fig. S1). Regression parameters are shown in Table 2. In this regression, only cytokine PC1 scores and PSS scores were positive predictors of being in the iHUD category. Overall, the multiple regression had a near unity AUROC (98.3; 95% CI:95.4–100; p < 0.0001), indicating excellent effectiveness in differentiating iHUD from HC [55]. In a follow-up sensitivity analysis, we examined the performance of the above multiple logistic regression, using only the n = 17 participants with methadone maintenance (i.e., excluding n = 4 with buprenorphine). In this sensitivity analysis, cytokine PC1 scores remained a positive predictor of HUD, adjusting for the other variables as in the overall sample (not shown).
Correlations of cytokine PC1 scores with demographic and clinical variables
As shown in Supplementary Table S5, in all participants combined, cytokine PC1 scores were positively correlated with BMI (surviving FDR correction for multiple comparisons). In the iHUD, there were negative correlations of cytokine PC1 scores with BDI-II scores and age of first use of heroin, and a positive correlation with duration of abstinence (none survived FDR correction; see Supplementary Fig. S2). The daily methadone dose was not correlated with cytokine PC1 scores, suggesting that the robust difference in cytokine PC1 scores between iHUD and HC is unlikely to be primarily driven by methadone exposure per se. Years of regular alcohol or cannabis use were not correlated with cytokine PC1 scores. See the absence of correlations between PC1 scores and all other measures in Table 1, for iHUD, HC, and across both groups (Table S5).
Discussion
Using a representative panel of cytokines as well as inflammatory mediators, we found that 29 targets were significantly different between iHUD and HC (26 of these were higher in iHUD vs. HC). Importantly, these 29 targets included 19 members of several major cytokine families (chemokines, interleukins, growth factor, and TNF-related), showing robust dysregulation of different cytokine systems in HUD. Several studies, primarily in vitro, have shown mechanistic interactions between MOR systems and specific cytokine receptors [15, 56]. Furthermore, chronic exposure to MOR agonists during HUD causes a repeated disruption to the hypothalamic–pituitary–adrenal (HPA) stress axis [57, 58], and adrenal corticosteroids (e.g., cortisol) have a major modulatory role for diverse cytokines [59,60,61]. These are therefore two major types of mechanisms that could underlie robust differences in cytokine levels between iHUD and HC.
Cytokines with higher levels in iHUD vs. HC
Some of the cytokines that were elevated in the iHUD vs HC, such as the interleukin IL6, were previously reported [25, 47]. IL6 has primarily pro-inflammatory effects both in the central nervous system and peripherally [62, 63], and also regulates downstream cytokine networks [17]. Other cytokines that were elevated in iHUD vs HC, including the chemokine CCL2 (ligand for the CCR2 receptor), were recently shown to mediate neuro-glial adaptations after MOR-agonist exposure [18]. Furthermore, genes for several of the cytokines (or their cognate receptors) that were elevated in the iHUD versus HC exhibited changes in regional brain expression in rodents exposed to MOR agonists, including CCL2, hepatocyte growth factor (HGF), oncostatin M (OSM) and colony-stimulating factor-1 (CSF1) [24, 64, 65]. Overall, the specific functions and status of these targets as markers of disease severity in iHUD are important areas for future study.
Cytokines with lower levels in iHUD vs. HC
Only three of the cytokines in the assay were significantly lower in iHUD vs. HC: stem cell factor (SCF), interleukin IL-7, and the chemokine CCL28. A small number of studies have reported interactions between these cytokines and opioid receptor systems, either in vitro or in animal models [66,67,68]. However, to our knowledge, this is the first report to show differences in their serum levels in iHUD vs. HC. Therefore future studies should examine their potential mechanistic relevance to this disorder.
Other inflammatory targets (not part of canonical cytokine families)
Ten proteins that are not part of canonical cytokine families had higher levels in iHUD vs. HC (see Fig. 1). Functional changes in some of those proteins have been observed after MOR-agonist exposure (e.g., adenosine deaminase, caspase-8, eukaryotic translation initiation factor 4E-binding protein 1) in experimental settings [69,70,71]. The mechanistic underpinnings and consequences of changes in these inflammatory proteins in the context of HUD can be the focus of future studies.
Developing a “cytokine biomarker score” to differentiate iHUD from HC
In addition to documenting differences in specific serum cytokine and inflammatory proteins of iHUD vs. HC, this study also identified a robust multi-target blood-based biomarker score (based on PC1 scores from the 19 cytokines that differed between the groups). This score is of potential diagnostic value as a positive predictor of being in the HUD class since the univariate AUROC was 91.7%, considered in the “excellent” range [55]. The diagnostic value of this PC1 cytokine biomarker score survived adjustment for major variables known to affect cytokine markers (e.g., age, sex, BMI, and sleep) in a multiple logistic regression [31, 32, 72, 73]. In this multiple regression, the perceived stress score was also a positive predictor of being in the iHUD category. This finding is consistent with the role of stress exposure in the severity of HUD, and in dysregulation of the HPA-stress axis (which can itself affect cytokine systems) [21, 74]. In follow-up analyses, this cytokine PC1 score was correlated with specific clinical and age trajectory aspects in the iHUD (although these correlations did not survive FDR correction, in this relatively small sample). Specifically, in the iHUD, the cytokine PC1 score was negatively correlated with depression BDI-II scores and with the age of onset of regular heroin use. Depression signs and the age trajectory of heroin are important facets of HUD history and severity [29, 44]. Intriguingly, cytokine PC1 scores were positively correlated with the duration of heroin abstinence. However, for all these correlations, larger samples are necessary to determine the linearity of these relationships with cytokine PC1 scores (and with specific cytokines therein), while adjusting for demographic and clinical features.
Methodological considerations
We employed a simplified two-step approach for dimensionality reduction: first focusing on 29 targets that differed significantly between iHUD and HC, and secondly with PCA based on the subset of 19 targets that are members of canonical cytokine families [17, 25]. Other machine learning methods to elucidate the optimal components of a “cytokine PC score” for use in iHUD can also be evaluated in larger studies. Second, while circadian effects have been detected for specific cytokines (e.g., IL6) [75], the present samples were obtained across a relatively broad range in daytime hours. Nevertheless, this time range did not vary systematically across iHUD and HC, therefore, it is unlikely that the robust group differences are mainly driven by circadian effects. Third, as expected in a clinical inpatient population, the iHUD reported common therapeutic medications on the day of the blood sample (e.g., SSRI, trazodone, bupropion), whereas these were less frequent in the HC. Future larger studies may examine whether these medications have robust effects on the cytokine biomarker score. Fourth, due to the presence of “floor” effects in depression and anxiety scores in the HC, we opted not to adjust for these dimensions in the multivariable model. Intriguingly, a recent study using the same assay found that serum cytokine levels were unchanged in patients with depression and anhedonia as compared to controls [11]. Nevertheless, future studies could explore whether depression and/or anxiety processes affect these cytokine biomarker scores in HUD. Finally, because the iHUD were on stable daily MAT, and blood samples were collected at least 1 h after daily dosing, it is unlikely that withdrawal affected results (under these conditions it would not have been experienced to a substantial degree at the time of blood draw). This conclusion receives support from a clinical interview conducted at approximately the same time.
Future directions
A small number of recent studies suggest that differences in levels of specific cytokines can normalize over prolonged opioid abstinence, (e.g., for IL6) [25]. Therefore, future studies in larger cohorts should determine if this multi-target cytokine biomarker score (or other multi-target blood biomarker approaches) differs across stages in HUD recovery trajectory, including protracted abstinence, as well as based on disease severity. Another crucial avenue for future research involves investigating sex differences [1]. Studies have indicated that women with some substance use disorders exhibit heightened susceptibility to stress exposure, and this profile could potentially result in sexually dimorphic cytokine dysregulation [21, 72]; nevertheless, sex differences in cytokine responses to MOR agonists are yet to be investigated in depth in humans [21].
Conclusions and future studies
This is one of the few studies to examine a comprehensive set of cytokines from several major families, detecting robust differences in levels of both previously known and novel targets [21], in iHUD compared to HC. Cytokines and their cognate receptors are known to act in interactive networks, both in the periphery and brain [17]. In this regard, ours is also the first study to provide a multi-target ”cytokine biomarker score” [48, 76] that was a positive predictor of being in the iHUD group, surviving adjustment for major demographic and clinical variables. Since perceived stress scores were also positive predictors in the multivariable model, future larger-scale studies could determine whether stress mechanisms are directly related to the cytokine score differences observed between the groups [30]. Blood-based and other biomarkers are potentially important tools in research and in the care of psychiatric conditions, including substance use disorders [6, 8, 10, 77, 78]. Such blood biomarkers could eventually enhance clinical diagnostic interviews, providing a further mechanistically based understanding of risk stratification, trajectory, and treatment prognosis [6, 78].
Data availability
The main assay used here (Olink Target 96 Inflammation; is commercially available; www.olink.com). Statistical analysis routines are available upon request to scientific researchers. Data from this study are available upon request, after consultation with IRB.
References
Butelman ER, Huang Y, Epstein DH, Shaham Y, Goldstein RZ, Volkow ND, et al. Overdose mortality rates for opioids and stimulant drugs are substantially higher in men than in women: state-level analysis. Neuropsychopharmacology. 2023. https://doi.org/10.1038/s41386-023-01601-8.
Provisional Data Shows U.S. Drug Overdose Deaths Top 100,000 in 2022. In: Centers for Disease Control and Prevention [Internet]. Available: https://blogs.cdc.gov/nchs/2023/05/18/7365/.
Wakeman SE, Larochelle MR, Ameli O, Chaisson CE, McPheeters JT, Crown WH, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3:e1920622. https://doi.org/10.1001/jamanetworkopen.2019.20622.
Buonora M, Perez HR, Stumph J, Allen R, Nahvi S, Cunningham CO, et al. Medical record documentation about opioid tapering: examining benefit-to-harm framework and patient engagement. Pain Med. 2020;21:2574–82. https://doi.org/10.1093/pm/pnz361.
Glanz JM, Xu S, Narwaney KJ, McClure DL, Rinehart DJ, Ford MA, et al. Association between opioid dose reduction rates and overdose among patients prescribed long-term opioid therapy. Subst Abus. 2023. https://doi.org/10.1177/08897077231186216.
Volkow ND, Koob G, Baler R. Biomarkers in substance use disorders. ACS Chem Neurosci. 2015;6:522–5. https://doi.org/10.1021/acschemneuro.5b00067.
Morcuende A, Navarrete F, Nieto E, Manzanares J, Femenía T. Inflammatory Biomarkers in Addictive Disorders. Biomolecules. 2021;11:1824. https://doi.org/10.3390/biom11121824.
Dunn KE, Strain EC. Establishing a research agenda for the study and assessment of opioid withdrawal. Lancet Psychiatry. 2024. https://doi.org/10.1016/S2215-0366(24)00068-3.
Chapman BP, Gullapalli BT, Rahman T, Smelson D, Boyer EW, Carreiro S. Impact of individual and treatment characteristics on wearable sensor-based digital biomarkers of opioid use. NPJ Digit Med. 2022;5:123. https://doi.org/10.1038/s41746-022-00664-z.
Caspani G, Sebők V, Sultana N, Swann JR, Bailey A. Metabolic phenotyping of opioid and psychostimulant addiction: a novel approach for biomarker discovery and biochemical understanding of the disorder. Br J Pharmacol. 2022;179:1578–606. https://doi.org/10.1111/bph.15475.
Costi S, Morris LS, Collins A, Fernandez NF, Patel M, Xie H, et al. Peripheral immune cell reactivity and neural response to reward in patients with depression and anhedonia. Transl Psychiatry. 2021;11:565. https://doi.org/10.1038/s41398-021-01668-1.
Marchese S, Cancelmo L, Diab O, Cahn L, Aaronson C, Daskalakis NP, et al. Altered gene expression and PTSD symptom dimensions in World Trade Center responders. Mol Psychiatry. 2022;27:2225–46. https://doi.org/10.1038/s41380-022-01457-2.
Reeves KC, Shah N, Muñoz B, Atwood BK. Opioid receptor-mediated regulation of neurotransmission in the brain. Front Mol Neurosci. 2022;15:919773. https://doi.org/10.3389/fnmol.2022.919773.
Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993;44:8–12.
Eisenstein TK. The role of opioid receptors in immune system function. Front Immunol. 2019;10:2904. https://doi.org/10.3389/fimmu.2019.02904.
Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen L-Y. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483:175–86. https://doi.org/10.1016/j.ejphar.2003.10.033.
Becher B, Spath S, Goverman J. Cytokine networks in neuroinflammation. Nat Rev Immunol. 2017;17:49–59. https://doi.org/10.1038/nri.2016.123.
Zhu Y, Yan P, Wang R, Lai J, Tang H, Xiao X, et al. Opioid-induced fragile-like regulatory T cells contribute to withdrawal. Cell. 2023;186:591–606.e23. https://doi.org/10.1016/j.cell.2022.12.030.
Kruyer A, Angelis A, Garcia-Keller C, Li H, Kalivas PW. Plasticity in astrocyte subpopulations regulates heroin relapse. Sci Adv. 2022;8:eabo7044. https://doi.org/10.1126/sciadv.abo7044.
Hashimoto A, Kawamura N, Tarusawa E, Takeda I, Aoyama Y, Ohno N, et al. Microglia enable cross-modal plasticity by removing inhibitory synapses. Cell Rep. 2023;42:112383. https://doi.org/10.1016/j.celrep.2023.112383.
Butelman ER, Goldstein RZ, Nwaneshiudu CA, Girdhar K, Roussos P, Russo SJ, et al. Neuroimmune mechanisms of opioid use disorder and recovery: Translatability to human studies, and future research directions. Neuroscience. 2023. https://doi.org/10.1016/j.neuroscience.2023.07.031.
Hofford RS, Russo SJ, Kiraly DD. Neuroimmune mechanisms of psychostimulant and opioid use disorders. Eur J Neurosci. 2019;50:2562–73. https://doi.org/10.1111/ejn.14143.
Marchette RCN, Carlson ER, Said N, Koob GF, Vendruscolo LF. Extended access to fentanyl vapor self-administration leads to addiction-like behaviors in mice: blood chemokine/cytokine levels as potential biomarkers. Addict Neurosci. 2023;5. https://doi.org/10.1016/j.addicn.2022.100057.
Zhang Y, Liang Y, Levran O, Randesi M, Yuferov V, Zhao C, et al. Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology. 2017;234:2259–75. https://doi.org/10.1007/s00213-017-4657-y.
Re G-F, Jia J, Xu Y, Zhang Z, Xie Z-R, Kong D, et al. Dynamics and correlations in multiplex immune profiling reveal persistent immune inflammation in male drug users after withdrawal. Int Immunopharmacol. 2022;107:108696. https://doi.org/10.1016/j.intimp.2022.108696.
Salarian A, Kadkhodaee M, Zahmatkesh M, Seifi B, Bakhshi E, Akhondzadeh S, et al. Opioid use disorder induces oxidative stress and inflammation: the attenuating effect of methadone maintenance treatment. Iran J Psychiatry. 2018;13:46–54. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5994232.
Zhang Z, Wu H, Peng Q, Xie Z, Chen F, Ma Y, et al. Integration of molecular inflammatory interactome analyses reveals dynamics of circulating cytokines and extracellular vesicle long non-coding RNAs and mRNAs in heroin addicts during acute and protracted withdrawal. Front Immunol. 2021;12:730300. https://doi.org/10.3389/fimmu.2021.730300.
Carlyle BC, Kitchen RR, Mattingly Z, Celia AM, Trombetta BA, Das S, et al. Technical performance evaluation of Olink Proximity Extension Assay for blood-based biomarker discovery in longitudinal studies of Alzheimer’s disease. Front Neurol. 2022;13:889647. https://doi.org/10.3389/fneur.2022.889647.
Butelman ER, Chen CY, Brown KG, Kreek MJ. Escalation of drug use in persons dually diagnosed with opioid and cocaine dependence: Gender comparison and dimensional predictors. Drug Alcohol Depend. 2019;205:107657. https://doi.org/10.1016/j.drugalcdep.2019.107657.
Ménard C, Pfau ML, Hodes GE, Russo SJ. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology. 2017;42:62–80. https://doi.org/10.1038/npp.2016.90.
Ter Horst R, Jaeger M, Smeekens SP, Oosting M, Swertz MA, Li Y, et al. Host and environmental factors influencing individual human cytokine responses. Cell. 2016;167:1111–.e13. https://doi.org/10.1016/j.cell.2016.10.018.
Schmidt FM, Weschenfelder J, Sander C, Minkwitz J, Thormann J, Chittka T, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS ONE. 2015;10:e0121971. https://doi.org/10.1371/journal.pone.0121971.
Wright KP Jr, Drake AL, Frey DJ, Fleshner M, Desouza CA, et al. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav Immun. 2015;47:24–34. https://doi.org/10.1016/j.bbi.2015.01.004.
Langstengel J, Yaggi HK. Sleep deficiency and opioid use disorder: trajectory, mechanisms, and interventions. Clin Chest Med. 2022;43:e1–e14. https://doi.org/10.1016/j.ccm.2022.05.001.
McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. https://doi.org/10.1016/0740-5472(92)90062-s.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. https://www.ncbi.nlm.nih.gov/pubmed/9881538.
Valdes-Marquez E, Clarke R, Hill M, Watkins H, Hopewell JC. Proteomic profiling identifies novel independent relationships between inflammatory proteins and myocardial infarction. Eur J Prev Cardiol. 2023;30:583–91. https://doi.org/10.1093/eurjpc/zwad020.
Jacquens A, Needham EJ, Zanier ER, Degos V, Gressens P, Menon D. Neuro-inflammation modulation and post-traumatic brain injury lesions: from bench to bed-side. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms231911193.
Park J, Jang W, Park HS, Park KH, Kwok S-K, Park S-H, et al. Cytokine clusters as potential diagnostic markers of disease activity and renal involvement in systemic lupus erythematosus. J Int Med Res. 2020;48:300060520926882. https://doi.org/10.1177/0300060520926882.
Liang J, Xu Y, Gao W, Sun Y, Zhang Y, Shan F, et al. Cytokine profile in first-episode drug-naïve major depressive disorder patients with or without anxiety. BMC Psychiatry. 2024;24:93. https://doi.org/10.1186/s12888-024-05536-2.
Cohen S. Perceived stress in a probability sample of the United States. The social psychology of health. 1988;251: 31–67. https://psycnet.apa.org/fulltext/1988-98838-002.pdf.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. https://www.ncbi.nlm.nih.gov/pubmed/6668417.
Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. https://doi.org/10.1207/s15327752jpa6703_13.
Butelman ER, Bacciardi S, Maremmani AGI, Darst-Campbell M, Correa da Rosa J, Kreek MJ. Can a rapid measure of self-exposure to drugs of abuse provide dimensional information on depression comorbidity? Am J Addict. 2017;26:632–9. https://doi.org/10.1111/ajad.12578.
Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7.
Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13:293–308. https://doi.org/10.3109/00952998709001515.
Chan YY, Yang SN, Lin JC, Chang JL, Lin JG, Lo WY. Inflammatory response in heroin addicts undergoing methadone maintenance treatment. Psychiatry Res. 2015;226:230–4. https://doi.org/10.1016/j.psychres.2014.12.053.
Lu R-B, Wang T-Y, Lee S-Y, Chen S-L, Chang Y-H, See Chen P, et al. Correlation between interleukin-6 levels and methadone maintenance therapy outcomes. Drug Alcohol Depend. 2019;204:107516. https://doi.org/10.1016/j.drugalcdep.2019.06.018.
Struglics A, Larsson S, Lohmander LS, Swärd P. Technical performance of a proximity extension assay inflammation biomarker panel with synovial fluid. Osteoarthr Cartil Open. 2022;4:100293. https://doi.org/10.1016/j.ocarto.2022.100293.
Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300.
Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments. Philos Trans A Math Phys Eng Sci. 2016;374:20150202. https://doi.org/10.1098/rsta.2015.0202.
Josse J, Husson F. missMDA: a package for handling missing values in multivariate data analysis. J Stat Softw. 2016;70:1–31. https://doi.org/10.18637/jss.v070.i01.
Liu J, Chen B, Lu H, Chen Q, Li J-C. Identification of novel candidate biomarkers for acute myocardial infarction by the Olink proteomics platform. Clin Chim Acta. 2023;548:117506. https://doi.org/10.1016/j.cca.2023.117506.
Drożdż K, Nabrdalik K, Kwiendacz H, Hendel M, Olejarz A, Tomasik A, et al. Risk factors for cardiovascular disease in patients with metabolic-associated fatty liver disease: a machine learning approach. Cardiovasc Diabetol. 2022;21:240. https://doi.org/10.1186/s12933-022-01672-9.
Nahm FS. Receiver operating characteristic curve: overview and practical use for clinicians. Korean J Anesthesiol. 2022;75:25–36. https://doi.org/10.4097/kja.21209.
Rogers TJ. Bidirectional regulation of opioid and chemokine function. Front Immunol. 2020;11:94. https://doi.org/10.3389/fimmu.2020.00094.
Zhang G-F, Ren Y-P, Sheng L-X, Chi Y, Du W-J, Guo S, et al. Dysfunction of the hypothalamic-pituitary-adrenal axis in opioid dependent subjects: effects of acute and protracted abstinence. Am J Drug Alcohol Abuse. 2008;34:760–8. https://doi.org/10.1080/00952990802385781.
Li S-X, Shi J, Epstein DH, Wang X, Zhang X-L, Bao Y-P, et al. Circadian alteration in neurobiology during 30 days of abstinence in heroin users. Biol Psychiatry. 2009;65:905–12. https://doi.org/10.1016/j.biopsych.2008.11.025.
Dunn AJ. The HPA axis and the immune system: a perspective. In: NeuroImmune biology. Elsevier; 2007. pp. 3–15. https://doi.org/10.1016/S1567-7443(07)00201-3.
Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. 2009;16:300–17. https://doi.org/10.1159/000216188.
Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22:20–32. https://doi.org/10.1159/000362724.
Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. https://doi.org/10.1101/cshperspect.a016295.
Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–66. https://doi.org/10.7150/ijbs.4679.
Browne CJ, Futamura R, Minier-Toribio A, Hicks EM, Ramakrishnan A, Martínez-Rivera FJ, et al. Transcriptional signatures of heroin intake and relapse throughout the brain reward circuitry in male mice. Sci Adv. 2023;9:eadg8558. https://doi.org/10.1126/sciadv.adg8558.
Yan Y, Truitt B, Tao J, Boyles SM, Antoine D, Hulme W, et al. Single-cell profiling of glial cells from the mouse amygdala under opioid dependent and withdrawal states. iScience. 2023;26:108166. https://doi.org/10.1016/j.isci.2023.108166.
Pawlik K, Ciechanowska A, Ciapała K, Rojewska E, Makuch W, Mika J. Blockade of CC chemokine receptor type 3 diminishes pain and enhances opioid analgesic potency in a model of neuropathic pain. Front Immunol. 2021;12:781310. https://doi.org/10.3389/fimmu.2021.781310.
Zhang L, Belkowski JS, Briscoe T, Rogers TJ. Regulation of mu opioid receptor expression in developing T cells. J Neuroimmune Pharmacol. 2012;7:835–42. https://doi.org/10.1007/s11481-012-9396-6.
Brack A, Rittner HL, Machelska H, Beschmann K, Sitte N, Schäfer M, et al. Mobilization of opioid-containing polymorphonuclear cells by hematopoietic growth factors and influence on inflammatory pain. Anesthesiology. 2004;100:149–57. https://doi.org/10.1097/00000542-200401000-00024.
Nelson AM, Battersby AS, Baghdoyan HA, Lydic R. Opioid-induced decreases in rat brain adenosine levels are reversed by inhibiting adenosine deaminase. Anesthesiology. 2009;111:1327–33. https://doi.org/10.1097/ALN.0b013e3181bdf894.
Wang J, Charboneau R, Balasubramanian S, Barke RA, Loh HH, Roy S. Morphine modulates lymph node-derived T lymphocyte function: role of caspase-3, -8, and nitric oxide. J Leukoc Biol. 2001;70:527–36. https://www.ncbi.nlm.nih.gov/pubmed/11590188.
Ucha M, Coria SM, Núñez AE, Santos-Toscano R, Roura-Martínez D, Fernández-Ruiz J, et al. Morphine self-administration alters the expression of translational machinery genes in the amygdala of male Lewis rats. J Psychopharmacol. 2019;33:882–93. https://doi.org/10.1177/0269881119836206.
Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, et al. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol. 2012;27:156–66. https://doi.org/10.1002/hup.1251.
Liu Y-Z, Wang Y-X, Jiang C-L. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci. 2017;11:316. https://doi.org/10.3389/fnhum.2017.00316.
Kreek MJ, Reed B, Butelman ER. Current status of opioid addiction treatment and related preclinical research. Sci Adv. 2019;5:eaax9140. https://doi.org/10.1126/sciadv.aax9140.
Nilsonne G, Lekander M, Åkerstedt T, Axelsson J, Ingre M. Diurnal variation of circulating interleukin-6 in humans: a meta-analysis. PLoS ONE. 2016;11:e0165799. https://doi.org/10.1371/journal.pone.0165799.
Kuo H-W, Liu T-H, Tsou H-H, Hsu Y-T, Wang S-C, Fang C-P, et al. Inflammatory chemokine eotaxin-1 is correlated with age in heroin dependent patients under methadone maintenance therapy. Drug Alcohol Depend. 2018;183:19–24. https://doi.org/10.1016/j.drugalcdep.2017.10.014.
Goldstein RZ. Neuropsychoimaging measures as alternatives to drug use outcomes in clinical trials for addiction. JAMA Psychiatry. 2022 [cited 27 Jul 2022]. https://doi.org/10.1001/jamapsychiatry.2022.1970.
Byrne JF, Mongan D, Murphy J, Healy C, Fӧcking M, Cannon M, et al. Prognostic models predicting transition to psychotic disorder using blood-based biomarkers: a systematic review and critical appraisal. Transl Psychiatry. 2023;13:333. https://doi.org/10.1038/s41398-023-02623-y.
Acknowledgements
We are very grateful to all the clinical coordinators of the NARC laboratory.
Funding
This work was supported by NIDA U01DA053625 (ERB), R01DA049547 (NAK), R01DA047880 (PR), NCCIH R01AT010627 (RZG), NIMH R01MH104559 (SJR), R01MH127820 (SJR), and NIA R01AG067025 (PR).
Author information
Authors and Affiliations
Contributions
Study conception and design: (RZG, NAK, YH, ERB); data collection and sample preparation (YH, PR, FC); analysis and interpretation of results (FC, SJR, YH, ERB, NAK, RZG); draft manuscript preparation: (ERB, YH, NAK, RZG, POG). All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. Approval was obtained by the Icahn School of Medicine Institutional Review Board (IRB), protocol #19-00621. Informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Butelman, E.R., Huang, Y., Cathomas, F. et al. Serum cytokines and inflammatory proteins in individuals with heroin use disorder: potential mechanistically based biomarkers for diagnosis. Transl Psychiatry 14, 414 (2024). https://doi.org/10.1038/s41398-024-03119-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03119-z
This article is cited by
-
Brain microRNA profiles after exposure to heroin in rats
Experimental Brain Research (2025)