Abstract
Recent genome-wide association studies (GWASs) have identified fatty acid desaturase (FADS) genes, which code key enzymes involved in polyunsaturated fatty acid (PUFA) desaturation as susceptibility genes for bipolar disorder (BD). Several quantitative changes in PUFAs suggest their involvement in BD pathogenesis. Therefore, this study aimed to clarify the relationship between BD and PUFAs by conducting lipidomics covariating with the FADS gene variant (rs174550), which is associated with PUFA levels and BD susceptibility. The concentrations of 23 fatty acids were measured using plasma samples from the BD group (n = 535) and the control group (n = 107). Differences in each PUFA concentration ratio were compared between the two groups. Also, differences in each PUFA concentration ratio were compared for each genotype in rs174550. Our results showed that the BD group had significantly lower concentrations of linoleic acid (LA) (β = −0.36, p = 0.023) and arachidonic acid (AA) (β = −0.18, p = 0.013) than the control group. Concerning the effect of FADS on the PUFA concentration ratio, carriers of C-allele at rs174550 had significantly decreased γ-linolenic acid and AA concentration ratios. A previous GWAS reported that the presence of a C-allele at rs174550 increased the BD risk. This direction is consistent with the lipidomic results of the present study. In conclusion, both the FADS and BD were considered to regulate the AA concentration. Thus, as the FADS gene variant is crucial for conducting lipidomics of BD we believe that the allele frequency of FADS must be analyzed.
Similar content being viewed by others
Introduction
Bipolar disorder (BD) is characterized by mood swings, with alternating mania and depression. Among mental disorders, the heritability of BD is approximately 70–80%, as high as that of schizophrenia [1, 2]. Mood stabilizers such as lithium salts and second-generation antipsychotics are used to treat BD; however, their pharmacological mechanism has not yet been elucidated [3, 4]. Moreover, the pathophysiology of BD is still unclear; thus, its elucidation is essential for the development of new treatments and prevention of BD.
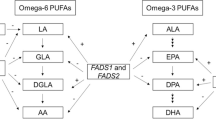
Polyunsaturated fatty acids (PUFAs, n-3 PUFAs including α-linolenic acid [ALA], eicosapentaenoic acid [EPA], and docosahexaenoic acid [DHA] and n-6 PUFAs including linoleic acid [LA], γ-linolenic acid [GLA], dihomo-γ-linolenic acid [DGLA], and arachidonic acid [AA]) are possible novel treatment options. This speculation is based on several epidemiological, clinical, biomarker, and genomic evidence. For example, Noaghiul et al. reported a negative correlation between fish consumption and the lifetime prevalence of BD [5]. In addition, clinical trials of PUFA supplementation in patients with BD have been conducted because n-3 PUFAs are safe and convenient to use. However, a clear conclusion has not been established. A recent meta-analysis showed that n-3 PUFA supplementation has beneficial effects on improving BD symptoms [6].
Lipidomics studies have reported that individuals with BD have a PUFA profile different from healthy controls [7,8,9,10,11,12,13,14] although reports on the association between BD and changes in PUFA levels are not always consistent: Chiu et al. reported low plasma concentrations of AA and DHA in patients with BD, whereas Evans et al. revealed no significant difference in the plasma concentrations of AA, EPA, and DHA between the BD group and the control group. Koga et al. analyzed the largest sample size, reporting high plasma concentrations of n-6 PUFAs such as AA and low levels of n-3 PUFAs such as EPA and DHA in patients with BD. Such conflicting results are possibly caused by the small samples analyzed in each study, introducing inadequate statistical power.
Each of the n-3 and n-6 PUFA was biosynthesized by fatty acid desaturase (FADS1 and FADS2). ALA was metabolized to EPA and DHA by the FADS enzyme. Similarly, LA was metabolized to GLA, DGLA, and AA by the FADS enzyme (Supplementary Fig. 1). Recent genome-wide association studies (GWASs) have identified fatty acid desaturase (FADS1 and FADS2) genes, which encode enzymes involved in n-3 and n-6 lipid biosynthesis pathways, as susceptibility genes of BD (top single nucleotide polymorphism [SNP] rs28456) [15, 16]. In addition, many studies of quantitative trait loci (QTL) have shown that FADS genes are associated with PUFA concentrations [17,18,19]. In particular, Drajoo et al. reported that the concentrations of n-6 PUFAs (LA, GLA, DGLA, and AA) and n-3 PUFAs (ALA and EPA) were significantly related to FADS gene variants (i.e., LA rs174547, GLA rs174546, DGLA, rs174548, AA rs174577, ALA rs509360, and EPA rs174538). The effect size of variants on increasing or decreasing PUFA levels is modest compared with that of susceptibility for complex disorders, including BD. Interestingly, specific alleles that are associated with each PUFA levels are in tight linkage disequilibrium with BD susceptibility variants (i.e., the G allele of rs28456), indicating that low AA levels may be associated with susceptibility to BD.
Taken together, we speculate that the inconsistent results of lipidomics for BD may be induced by not considering the QTL effect of FADS gene variants. This study aimed to use lipidomics with a FADS gene-variant polymorphism as a covariate to examine the relationship between BD and PUFAs in the largest sample to date.
Methods
Subjects
The study recruited a total of 535 participants in the BD group and 107 in the control group from Fujita Health University and its affiliated hospitals. In the BD group, 179 of 535 individuals presented with type 1 BD (BD1), 313 with type 2 BD (BD2), and 43 with schizoaffective disorder (SA). In terms of clinical status, 117 individuals were in a depressive state, 28 in a manic state, and 390 were in remission. BD was diagnosed based on the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) by at least two experienced psychiatrists using medical records and conducting unstructured interviews with the participants and their families. Individuals with known intellectual disabilities were excluded.
Written informed consent was obtained from all participants after they received a complete description of the study. This study was approved by the ethics committees of Fujita Health University and each participating hospital.
Fatty acid concentration measurement
Blood samples of the BD group were collected at the outpatient clinic, and plasma was extracted as soon as possible. In detail, vacuum tube blood collection was performed using Benoject II Vacuum Blood Collection Tubes VP-NA050K (containing EDTA-2Na), and 7 mL of blood was collected per tube. The blood samples were stored at 4 °C. To measure fatty acid concentrations, samples were centrifuged at 1000 g for 10 min at 4 °C to separate the plasma.
The separated plasma was stored at −80 °C, and the following 23 types of saturated, monounsaturated, and polyunsaturated fatty acids were analyzed using a triple quadrupole gas chromatograph mass spectrometer GCMS-TQ8040 (SHIMADZU CORPORATION Kyoto, Japan): ALA, EPA, DHA, LA, GLA, DGLA, AA, lauric acid, myristic acid, myristoleic acid, pentadecanoic acid, palmitic acid, palmitoleic acid, margaric acid, heptadecanoic acid, stearic acid, oleic acid, nonadecanic acid, arachidic acid, eicosenoic acid, eicosadienoic acid, behenic acid, and lignoceric acid.
To consider changes in the concentration of all fatty acids that fluctuate with diet and match the outcome of the previously reported QTL analysis [17,18,19], the fatty acid concentration ratio (each fatty acid concentration/total fatty acid concentration) was calculated from the measured fatty acid concentration. In this study, the n-3 and n-6 PUFAs (ALA, EPA, DHA, LA, GLA, DGLA, and AA) were measured and used as primary outcomes in the analysis. Further, other fatty acids were measured to calculate the concentration ratios.
Genotyping
Typing was performed using Illumina HumanOmniExpressExome 1.2 chip for 520 subjects of the BD group and 72 subjects of the controls group. In addition, 15 subjects of the BD group and 35 subjects of the control group were further genotyped for rs174550 (FADS gene) using the TaqMan probe method. rs174550 was selected because rs174550 and top SNPs of a previous report [18] are in nearly absolute linkage disequilibrium (i.e., LA rs174547, R2 = 0.999; GLA rs174546, R2 = 0.999; DGLA rs174548, R2 = 1.0; AA rs174577, R2 = 0.9372; ALA rs509360, R2 = 0.9785; EPA rs174538, R2 = 1.0).
Statistical analysis
First, a simple linear regression analysis was conducted using the diagnosis (BD group/control group) and rs174550 as independent variables. Next, multiple linear regression analysis was performed to compare the difference in PUFAs between the BD group and the control group. This analysis is the main analysis of this study. The ratio of each fatty acid (concentration of each PUFA/total fatty acid concentration) was used as the dependent variable. The independent variables included diagnosis (BD group /control group: primary outcome), sex, BMI, age, postprandial time (minutes), 4 °C storage time (minutes), hemolysis (yes/no), chyle (yes/no), dyslipidemia (yes/no), diabetes (yes/no), hypertension (yes/no), and rs174550 (CC/CT/TT). The level of significance was set at p < 0.05. The significance level for rs174550 was set at the level for the genome-wide significance (p < 5.0 × 10−8) because previous studies [15,16,17,18,19] referenced while selecting rs174550 were genome-wide association analyses; thus, we matched that level accordingly. Linear regression analyses were performed using R version 3.6.3.
Results
Table 1 shows participants’ backgrounds. Significant differences between the BD group and control group were observed in, age, postprandial time, BMI, sex, and dyslipidemia status, all of which were covariates in the main analysis. Supplementary Table 1 shows the fatty acid concentration ratios and concentrations analyzed. Supplementary Table 2 depicts the results of the simple linear regression analysis of PUFA concentration ratio and diagnosis, PUFA concentration ratio, and rs174550. Table 2 shows the results of the diagnosis (BD group/control group)—the primary outcome in this study—and of rs174550 (CC/CT/TT) in the multiple linear regression analysis. Supplementary Table 3 shows all multiple linear regression analysis results.
The diagnostic status was significantly associated with the concentration ratios of LA (β = −0.36, p = 0.023) and AA (β = −0.18, p = 0.013) in multiple linear regression analysis. These results indicated that the BD group had significantly lower LA and AA concentration ratios. For other PUFAs, no association was found between BD diagnosis and concentration ratios.
The QTL SNP (rs174550), which was reported in previous studies [17,18,19], was significantly associated with the concentration ratios of GLA (β = −0.079, p < 2.0 × 10−16) and AA (β = −0. 36, p < 2.0 × 10−16). These mean that patients with the C-allele in rs174550, which was a risk allele of BD, had significantly lower concentration ratios of GLA and AA than those with the T-allele. However, compared with the findings of previous studies [17,18,19], other PUFAs were not associated with the genotypes in rs174550.
Discussion
In this study, the BD group had significantly lower concentration ratios of LA and AA based on the analysis that took into account the QTL effect of SNP. This indicates the independent diagnostic effect of specific PUFAs on BD susceptibility. Furthermore, we conducted an ad hoc analysis to determine if the associations between PUFA and diagnosis differed depending on the BD subtype (BD1/BD2/SA) or status (depressive/manic/remission). Supplementary method 1 presents the detailed analysis methods. These results are shown in Supplementary Tables 4 and 5. Regarding the association between subtypes and LA, significant associations were found for BD1 and SA, with the same effect direction as observed in the overall BD group. Although BD2 was not significant, it showed the same effect direction. For AA, all subtypes showed significant associations with the same effect direction as observed in the overall BD group. Regarding the association between statuses and LA, significant associations were observed for all statuses, with the same effect direction as observed in the overall BD group. Regarding AA, significant associations were found in remission and manic statuses, with the same effect direction as observed in the overall BD group. Although the association with depressive status was not significant, it showed the same effect direction. Considerably, the effects of LA and AA observed in the overall BD group analysis are probably common across all BD subtypes or statuses.
In this study, we replicated the QTL effect on the concentrations of AA and GLA by the FADS SNP (rs174550), which was reported in various ancestries (Table 3) [17,18,19], although previous studies have shown that SNPs were associated with the concentrations of most PUFAs consisting of n-3 and n-6.
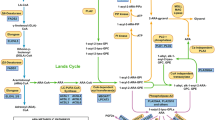
In the previous GWAS targeting BD [15], the C-allele of rs174550 was the risk allele for BD (OR = 1.17, p = 1.2 × 10−8). This finding supported the result that low GLA and AA concentrations in individuals harboring the C-allele were associated with BD, although other PUFAs, such as DHA and EPA, were not risk factors for BD. This also clarified the finding that BD risk by low AA concentration was explained both by the QTL effect of the SNP (decreased AA by genotype) and the diagnostic effect, which were independent of each other (AA, β = 0.070, p = 0.45; Fig. 1). By contrast, the low LA concentration was not influenced by the QTL effect but is mainly explained by the diagnostic effect. Such “non-significant” QTL effect is reasonable because LA concentration is mainly determined by diet (i.e., LA cannot be synthesized in the body), is in the upstream of the n-6 pathway, and is metabolized into GLA (significant QTL effect), DGLA, and AA (significant QTL effect) by FADS.
AA arachidonic acid, BD bipolar disorder. The whiskers at the top/bottom represent the maximum/minimum value excluding outliers. The line that divides the box into two parts represents the median. The bottom of the box represents the first quartile. The top of the box represents the third quartile. The dots represent outliers. This figure shows that both the QTL effect of SNP (reduction in AA by genotype) and the diagnostic effect are independently involved in the BD risk by low AA concentration.
Compared with previous lipidomics studies of BD (Table 4), the sample size in the present study is one of the largest and thus provided the most powerful results. However, we could not obtain concordant and conclusive results similar to other studies because the sample size was not large enough to deny type I and II errors. In addition, previous studies did not consider the QTL effect of the FADS SNP, which is also a susceptible SNP to BD. Such a small sample size induces variable allele frequencies either in cases or controls, which may influence the QTL effect on PUFAs. Therefore, the QTL effect is one of the most important confounding factors when interpreting the lipidomics for BD.
The strength of this study is attributed to the largest sample size, and the “abnormality” of AA concentration is a good reason to explore the causal effect on BD susceptibility because the QTL effect was concordant with those in other QTL studies [17,18,19]. Because QTLs in other PUFAs, except GLA and AA, were not replicated in this study, we speculated the following reasons: (1) the sample size was small to detect the QTL effect and (2) previous QTL results showed that the direction of the effect on DGLA in rs174550 was contrasting between Caucasians and Han Chinese, which suggest population difference in the effect of rs174550 on DGLA. However, as mentioned above, we replicated the QTL effect of the SNP on AA and GLA, concordant with other large QTL analyses, and thus concluded concrete results at least for AA.
We believe that AA is important in elucidating the pathogenesis of BD because several associations between AA metabolism and BD were reported so far [20]. According to reports, antipsychotics, and mood stabilizers such as lithium salts, valproate, carbamazepine, and lamotrigine affected AA metabolism in the brain via N-methyl-D-aspartate receptors and D2 receptors, which the authors called the “arachidonic cascade hypothesis.” In this hypothesis, plasma AA is consumed in BD because of increased AA metabolism in the brain, and mood stabilizers decrease the turnover of AA in several brain phospholipids. Even in accordance with this hypothesis, as our study revealed compatible results that the plasma AA concentrations decreased, it supported the finding that impaired AA metabolism affects the pathogenesis of BD. Therefore, further studies investigating AA metabolism in the brain are essential and may elucidate the pathogenesis of BD.
To interpret this result, we stress important limitations. We could not control the effect of diet. Although we included the time from the last diet as a covariate in the model and applied the ratio (e.g., AA/all PUFAs) to control the diet, we could not follow the contents of the diet, and such is associated with PUFAs located in the upstream of this pathway (such as LA). Further studies should be performed to examine dietary intake and items that can be included in multiple linear regression analyses. Moreover, the effects of dyslipidemia and diabetes medications on PUFA concentrations have not been elucidated. However, these medications are important for the evaluation of PUFA levels. This is because dyslipidemia medications can affect cholesterol and triglyceride levels, and some diabetes treatments can affect lipid levels [21]. Supplementary Tables 6 and 7 present the breakdown of dyslipidemia and diabetes medications. We included information on dyslipidemia and diabetes in our analysis as they may influence blood lipid levels. However, treatment medications were not included in the analysis. This is because most patients with dyslipidemia were taking treatment medications. Similarly, most patients with diabetes were also on their medication. Treatment medication information was excluded from the analysis due to the strong correlation between dyslipidemia and its treatment medication (r = 0.97), as well as diabetes and its treatment medication (r = 0.95). To examine the impact of dyslipidemia and diabetes medications on PUFA concentrations, further research specifically targeting these medications must be performed. In addition, several participants in the BD group were taking mood stabilizers, antipsychotics, or both in this study. Therefore, the effects of these medications on PUFA were examined in this study. Supplementary Table 8 shows the types of mood stabilizers and antipsychotics used. To examine the effects of these medications on PUFA, a multiple linear regression analysis was conducted using groups taking antipsychotics (AP), lithium salts (Li), and anticonvulsants (AC) and a group not taking any of these medications. Supplementary method 2 and Supplementary Table 9 present the detailed analysis methods and results. The group not taking any of these medications was considered the reference group (no-medication group). The beta (β) coefficients of the concentration ratio of PUFA in groups taking AP (AP group), Li (Li group), AC (AC group), both AP and Li (AP + Li group), AP and AC (AP + AC group), Li and AC (Li + AC group) and AP, Li, and AC (AP + Li + AC group) were calculated. In particular, we focused on LA and AA, where significant associations were observed in the main analysis under the diagnostic categories of the independent variables. For LA, the AP and Li groups had positive β coefficients. The AC group and the two or more medications group had negative β coefficients, with a relatively large β coefficient and significant association observed particularly in the AP + Li + AC group. This finding indicates that the concomitant use of AP, Li, and AC decreased the LA concentration ratios. The effect of the diagnosis in reducing the LA concentration ratios observed in the main analysis may have been enhanced by the effect of reducing the LA concentration ratios present in the two or more medications group. However, understanding why a negative β coefficient was observed in the AP + Li group despite the positive β coefficients in the AP and Li groups, as well as why this trend is most pronounced in the AP + Li + AC group, is difficult. Furthermore, no studies have reported a relationship between these medications and LA so far. The effects of medications used in BD on LA should be clarified in future research with larger sample sizes. Regarding AA, there were no notably large β coefficients among the groups, nor were there any significant associations with AA. Therefore, the relationship between medications and AA was not clarified.
In conclusion, the BD group has significantly lower AA and LA concentration ratios than the control group. In addition, a strong QTL effect of the FADS SNP was found on AA and GLA; however, such an effect was independent of BD susceptibility. Therefore, AA and LA are good candidates for susceptibility to BD, and such QTL effect of the SNP should be included as a covariate to interpret the lipidomics for BD.
Data availability
All data relevant to the study are included in the article or uploaded as online supplementary information. The data generated in this study will be available from the corresponding author on reasonable request.
References
Müller WE, Müller JK. Basic data for bipolar disorders: genetics, neurobiology and pharmacology. Med Monatsschr Pharm. 2016;39:371–6.
Mclntyre RS, Berk M, Brietzke E, Goldstein BI, López-Jaramillo C, Kessing LV, et al. Bipolar Disorders. Lancet. 2020;396:1841–56.
Jung GL, Young SW, Sung WP, Dae-Hyun S, Mi KS, Won-Myong B. Neuromolecular Etiology of Bipolar Disorder: Possible Therapeutic Targets of Mood Stabilizers. Clin Psychopharmacol Neurosci. 2022;31:228–39.
Tadafumi K. Current understanding of bipolar disorder: Toward integration of biological basis and treatment strategies. Psychiatry Clin Neurosci. 2019;73:526–40.
Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipoloar disorders. Am J Psychiatry. 2003;160:2222–7.
Taro K, Kenji S, Makoto O, Ikeda M, Iwata N. Omega-3 fatty acids for treating residual depressive symptoms in adult patients with bipolar disorder: a systematic review and meta-analysis of double-blind randomized, placebo-controlled trials. Bipolar Disord. 2021;23:730–1.
Chiu CC, Huang SY, Su KP, Lu ML, Huang MC, Chen CC, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13:99–103.
Sublette ME, Bosetti F, DeMar JC, Ma K, Bell JM, Fagin‐Jones S, et al. Plasma free polyunsaturated fatty acid level are associated with symptom severity in acte mania. Bipolar Disord. 2007;9:759–65.
Clayton EH, Hanstock TL, Hirneth S, Kable CJ, Garg ML, Hazell PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. 2008;43:1031–8.
McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126:303–11.
Evans SJ, Kamali M, Prossin AR, Harrington GJ, Ellingrod VL, McInnis MG, et al. Association of plasma n-3 and n-6 lipids with burden of disease measures in bipolar subjects. J Psychiatr Res. 2013;46:1435–41.
Saunders EF, Reider A, Singh G, Gelenberg AJ, Rapoport SI. Low unesterified: esterified eicosapentaenoic acid (EPA) plasma concentration ratio is associated with bipolar disorder episodes, and omega-3 plasma concentrations are altered by treatment. Bipolar Disord. 2015;17:729–42.
Koga N, Ogura J, Yoshida F, Hattori K, Hori H, Aizawa E, et al. Altered polyunsaturated fatty acid levels in relation to proinflammatory cytokines, fatty acid desaturase genotype, and diet in bipolar disorder. Transl Psychiatry. 2019;9:208.
Pomponi M, Janiri L, Torre GL, Stasio ED, Nicola MD, Mazza M. et al. Plasma levels of n-3 fatty acids in bipolar patients: deficit restricted to DHA. J Psychiatr Res. 2013;47:337–42.
Ikeda M, Takahashi A, Kamatani Y, Okahisa Y, Kunugi H, Mori N, et al. A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol Psychiatry. 2018;23:639–47.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JR, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PloS Genet. 2009;5:e1000338.
Dorajoo R, Sun Y, Han Y, Ke T, Burger A, Chang X, et al. A genome-wide association study of n-3 and n-6 plasma fatty acids in a Singaporean Chinese population. Genes Nutr. 2015;10:53.
Guan W, Steffen BT, Lemaitre RN, Wu JH, Tanaka T, Manichaikul A, et al. Genome-wide association study of plasma N6 polyunsaturated fatty acids within the cohorts for heart and aging research in genomic epidemiology consortium. Circ Cardiovasc Genet. 2014;7:321–31.
Stanley IR. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem Neurosci. 2014;5:459–67.
Sophia D, Ahmed KS, Tamim OA. Effects of novel glucose-lowering drugs on the lipid parameters: A systematic review and meta-analysis. Ann Med Surg. 2022;77:103633.
Acknowledgements
The authors thank all patients who participated in this study. We also thank Prof. Takehiko Yokomizo (Department of Biochemistry, Juntendo University School of Medicine), and Prof. Hyeon-Cheol Lee-Okada (Department of Biochemistry, Juntendo University School of Medicine) for their thoughtful comments and MARUZEN-YUSHODO Co., Ltd. (https://kw.maruzen.co.jp/kousei-honyaku/) for the English language editing. This study was supported by the SRPBS from the Japan Agency for Medical Research and Development (AMED) under Grant Numbers JP22wm0425008 (NI, MI, and TS) and JP22wm0525024 (MI); JSPS Kakenhi Grant Numbers JP21H02854 (MI), JP21K07490 (TS) and JP22H03003(NI); the Private University Research Branding Project from MEXT (NI). The funders played no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
TA, TS, MI, and NI conceived and designed the study. TA, TS, TO, KN, KI, and RA collected the samples. TA processed the samples and managed the data. TA and MI performed the experiments and analyzed the data. All authors contributed to the interpretation of the results. TA drafted the first version of the manuscript. TS, MI, and NI reviewed and edited the manuscript. All authors provided critical comments and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
Written informed consent was obtained from each subject after the procedures had been fully explained. This study was performed in accordance with the World Medical Association’s Declaration of Helsinki and approved by the Ethics Committees of Fujita Health University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ashizawa, T., Saito, T., Okochi, T. et al. Association of plasma arachidonic acid levels with a bipolar disorder and the effects of a FADS gene variant. Transl Psychiatry 14, 435 (2024). https://doi.org/10.1038/s41398-024-03141-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-024-03141-1
This article is cited by
-
The Effects and Mechanisms of n-3 and n-6 Polyunsaturated Fatty Acids in the Central Nervous System
Cellular and Molecular Neurobiology (2025)