Abstract
Biological aging has been linked to multiple psychological disorders, yet its extrapolation to suicide remains absent. We aimed to examine the associations of biological aging with suicidal ideation (SI) and suicide attempt (SA) and to explore possible moderators of the associations. A total of 124,529 middle and older participants from the UK Biobank were included. Phenotypic age (PhenoAge) indicating biological aging was calculated based on chronological age and nine clinical biomarkers. The residuals of PhenoAge regressed on chronological age were utilized to quantify biological aging, termed PhenoAge acceleration (PAA). Approximately one-third of baseline participants completed mental health follow-up questionnaires including suicide-related information. Multivariate logistic regression models were performed to estimate the associations. 2718 (2.2%) SA and 5207 (4.4%) SI cases were documented. Compared with participants in the lowest quartile of PAA, those in the highest quartile had a 21.8% [odds ratio (OR) = 1.218; 95% confidence interval (CI): 1.087–1.198) and 12.5% (OR = 1.125, 95% CI: 1.035–1.224) higher odds of SA and SI, respectively. Biologically older participants (PAA > 0) were more likely to report SA (OR = 1.104, 95% CI: 1.018–1.198) and SI (OR = 1.064, 95% CI: 1.003–1.129). Gender, age, socioeconomic status (SES), physical activity, and somatic and psychiatric disorders could modify the associations (P for interaction <0.05). Our findings indicated that PAA-measured aging might be positively associated with SA/SI. Interventions aimed at slowing aging might contribute to suicide prevention, especially among males, young adults, low SES, the physically inactive, and vulnerable populations.
Similar content being viewed by others
Introduction
Annually, over 700,000 individuals globally succumb to suicide, comprising 1.3% of the total mortality [1]. As the leading risk factors for suicide deaths, suicide attempts (SA) and suicidal ideation (SI) place a profound burden on society and have emerged as a significant public health crisis. Data from the World Health Organization (WHO) revealed that each suicide death is preceded by more than twenty SA [2]. Individuals who reported SI in the past 1 year had a significantly higher prevalence of future suicide [3]. Identifying emerging risk factors for SA and SI is paramount in the effort to prevent suicide deaths. While chronological age has been extensively studied as risk factors for suicide, biological age might offer unique insights by reflecting physiological processes, such as inflammatory response, metabolic health, and immune function [4, 5].
Biological age has been demonstrated as a better predictor of aging-related adverse outcomes than chronological age [6, 7]. To date, a variety of measurements of biological age have been proposed, ranging from biomarkers at the individual level to the integration of multi-omics aging clocks [4,5,6,7,8]. Among these, Phenotypic age (PhenoAge), a clinical biomarker-based measure of biological age [5, 9], has been developed and independently validated to predict morbidity and mortality in diverse populations [6, 10]. Although previous studies have demonstrated a possible association between suicide and biological aging, almost all studies to date have examined whether suicide may lead to accelerated biological aging [11,12,13,14,15]. However, no study has yet investigated whether biological aging itself can serve as a predictor of suicide. Notably, a recent large population-based cohort study has pointed to biological aging itself as a risk factor for anxiety/depression, which is a recognized risk factor for suicide [16]. It is reasonable to hypothesize that biological aging may be a potential risk factor for SA/SI. Biological aging might influence suicidal behaviors through mechanisms such as dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis [17, 18], systemic inflammatory disorders [19, 20], and stress-related hormonal disturbances [21, 22]. Additionally, PhenoAge acceleration (PAA), quantifying the difference between PhenoAge and chronological age, has been reported to perform well in differentiating the risk of several adverse outcomes [10, 16, 23]. Hence, investigating the role of PAA in suicidal behaviors could be instrumental in providing new perspectives on suicide prevention.
To characterize extant literature gaps, we conducted a prospective cohort study to examine the associations of PAA-measured biological aging with SA/SI and to explore whether the associations are modified by sociodemographic, lifestyle, and health-related factors.
Materials and methods
Study participants
The current study utilized data from the UK Biobank, one of Europe’s largest prospective cohorts with continuous follow-up, recruiting over 500,000 middle-aged and elderly participants (aged 37–73 years) in the United Kingdom from 2006–2010. In brief, participants in the UK Biobank provided information on the sociodemographic, lifestyle, environmental, and genetic determinants of a range of complex conditions through completing touchscreen questionnaires and medical examinations. A thorough description of the cohort was documented in earlier research [24]. To ensure the accuracy and reliability of the collected data, the UK Biobank implemented rigorous quality control measures across all stages of data collection, including standardized procedures for biological sample collection, data entry, and physical measurements. The biological samples, such as blood, urine, and saliva, were processed and stored under strict protocols to prevent degradation, with biochemical analyses conducted using validated laboratory techniques [25]. Additionally, extensive data linkage with electronic health records from the National Health Service (NHS) ensures the reliability of disease information. Informed consent was obtained from all participants before recruitment. The UK Biobank project received ethical approval from the Northwest Multi-center Research Ethics Committee (http://www.ukbiobank.ac.uk/ethics/). The authors declare that all procedures in this study were in accordance with the Declaration of Helsinki.
Between 2016 and 2017, about one-third of the initial participants (n = 157,366) completed a mental health questionnaire that contained information related to suicide [26]. Of these, 1922 participants who did not provide comprehensive data on SA/SI were excluded from this study. Second, participants who did not have the complete biomarker data required to calculate PhenoAge were omitted (n = 28,078). Furthermore, we excluded participants missing information on covariates (n = 2837). Finally, a total of 124,529 eligible participants were included in the formal analysis (Supplementary Fig. 1).
Assessment of exposures
Consistent with previous well-validated UK Biobank studies [10, 16, 23], PhenoAge was calculated using the Gompertz proportional hazard model [5], which integrates all-cause mortality prediction scores derived from nine clinical biomarkers—namely albumin, alkaline phosphatase, creatinine, glucose, C-reactive protein, lymphocyte percentage, mean cell volume, red cell distribution width, and white blood cell count—and chronological age (‘BioAge’ package in R software). The missing information for these biomarkers in this study and the corresponding Field IDs in the UK Biobank are shown in Supplementary Table 1. The percentage of missing data for these biomarkers ranged from 4.1% for red blood cell distribution width to 14.1% for glucose. To quantify biological aging, a linear regression model was applied to regress PhenoAge on chronological age, thereby calculating the residual value, referred to as PAA. PAA greater than zero were defined as indicating individuals who are biologically older than their chronological age. A detailed description of PhenoAge is outlined in Supplementary Methods.
Definition of outcome
In the UK Biobank’s online mental health follow-up survey (2016–2017), participants first responded to the question, “Have you deliberately harmed yourself, whether or not you meant to end your life?” (Data-Field: 20480). If the response was affirmative, they then were probed, “Have you harmed yourself with the intention to end your life?” (Data-Field: 20483). Responses of ‘no’ to either question resulted in a score of 0, signifying the absence of SA, and ‘yes’ to both led to a score of 1, indicating the presence of SA. SI was defined by the question, “How often have you been bothered by the thoughts that you would be better off dead or of hurting yourself in some way?” (Data-Field: 20513). Participants who answered ‘not at all’ were considered to be free of SI, otherwise, they did. The definition of SA and SI employed in the study has been confirmed by prior studies [27, 28].
Assessment of covariates
We considered sociodemographic characteristics [including chronological age (continuous), sex, ethnicity (white vs. others), educational level (college or university vs. others), employment status (employed vs. unemployed), and Townsend Deprivation Index (TDI, continuous)], lifestyle factors [including smoking, alcohol consumption, physical activity (PA), sleep duration, and body mass index (BMI)], and health-related factors [including hypertension, diabetes, coronary heart disease (CHD), stroke, and psychiatric disorders] as potential covariates. Chronological age was determined by subtracting the date of birth from the date of attending the baseline recruitment. The TDI covered information on social class, employment and housing, and reflected area-level deprivation, with higher scores indicating higher deprivation [29]. Based on previous studies [29,30,31], we stratified each lifestyle factor into healthy and unhealthy categories. The unhealthy categories encompassed current smokers, once or more per week of alcohol consumption, <7 or >9 h/day of sleep duration, BMI ≥ 25 kg/m2, and <150 min/week of moderate-intensity PA or <75 min/week of vigorous-intensity PA. Details on the assessment of lifestyle factors are presented in Supplementary Table 2. Psychiatric disorders include depression, anxiety, bipolar disorder (BD), and schizophrenia at baseline. Health-related factors were identified by the International Classification of Diseases, Tenth Revision (ICD-10) codes among the “First occurrence fields” of health-related outcomes from the UK Biobank (Data Category: 1712). Missing information and Field IDs in the UK Biobank for covariates are provided in Supplementary Table 3. The proportion of missing data for all covariates was less than 1%, with the highest being PA (0.8%).
Statistical analyses
ANOVA or Wilcoxon rank-sum tests were performed for continuous variables [expressed as mean (standard deviation, SD) or median (interquartile range, IQR)], and Chi-square tests were utilized for categorical variables [presented as frequency (%)], to describe baseline characteristics by biological aging or SA/SI. Multivariable logistic regression models were utilized to explore the associations of PAA with the odds of SA/SI, with results presented as odds ratios (OR) and 95% confidence intervals (CI). Three models with stepwise adjustments for covariates were conducted: Model 1 adjusted for sociodemographic factors; Model 2 accounted for lifestyle factors plus Model 1; Model 3 augmented Model 2 with health-related factors. First, to estimate the odds of SA/SI ascribed to high PAA, we analyzed PAA as categorical variables (Quartiles), considering the lowest PAA (Quartile 1) as the reference group. The trend tests were conducted by designating quartiles as continuous variables. Subsequently, the PAA was standardized and processed as a continuous variable to calculate OR per SD increment. Also, to assess the impact of PAA on SA/SI in participants with the same chronological age, we calculated the OR for biologically older participants, using biologically younger as the reference group. The restricted cubic spline (RCS) (‘rcssci’ package in R software) model was applied to investigate the dose-response relationship between PAA and SA/SI [32].
To explore whether possible moderators of the associations between PAA and SA/SI, subgroup analyses were performed by sex (male vs. female), age (<60 years vs. ≥60 years), education (college or university vs. others), TDI (<median vs. ≥median), employment status (being employed vs. others), lifestyles (healthy vs. unhealthy), and health-related factors (yes vs. no). Likelihood tests were conducted to test the significance of the moderation effect by including or excluding the interaction term between PAA and the stratification factor in Model 3.
To augment the robustness of the findings, several sensitive analyses were conducted. First, considering that childhood adversity may influence the relationship between the exposure of interest and SA [33, 34], the study further adjusted for childhood adversity based on Model 3. Childhood adversity was evaluated using the Childhood Trauma Screen, a widely used online scale including physical abuse and neglect, emotional abuse and neglect, and sexual abuse [33]. Detailed evaluations of childhood adversity can be found in Supplementary Table 4. Second, we reran the primary analysis after filling in covariates with missing values using multiple imputation techniques (‘mice’ package in R software). Third, we reanalyzed the associations without adjusting for psychiatric disorders or excluding individuals with psychiatric disorders at baseline. Fourth, we reran the association analyses after further adjustment for neurological disorders, including dementia and Parkinson’s disease. Fifth, to minimize the effect of reverse causation bias, we reran the analyses after excluding participants who self-reported suicide attempts or self-harm (UK Biobank Field ID: 20002) at baseline. Sixth, we re-examined the associations using the quintile categorization of PAA. Seventh, we additionally adjusted for the effects of cancer at baseline. Eighth, the associations were reanalyzed after excluding patients with depression or cardiovascular diseases (CVD, including CHD and stroke) at baseline.
All analyses were conducted by SAS 9.4 (SAS Institute, Cary, NC, USA) and R Statistical Software (version 4.2.2). A two-tailed P value < 0.05 was regarded as statistically significant.
Results
Baseline characteristics
The study comprised 124,529 participants [mean age (SD), 55.9 (7.7) years; 44.2% were male], and 2718 (2.2%) SA and 5207 (4.2%) SI cases were recorded at follow-up. 39.6% of participants were biologically older. The biologically younger and older groups had nearly identical mean chronological ages, yet they exhibited a mean biological age disparity of 6.6 years. Biologically older individuals were more likely to be in the age group 60 and older. Compared with biologically younger individuals, those who were biologically older tended to be male, non-white, have lower socioeconomic status (SES, including less educated and unemployment), face high levels of poverty, possess unhealthy lifestyles, and suffer from physical or psychiatric disorders at baseline (Table 1). Similar findings were obtained when describing baseline characteristics stratified by SA or SI (Supplementary Table 5).
Longitudinal associations of PAA with SA and SI
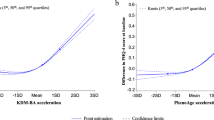
RCS analysis did not reveal a nonlinear association between PAA and SA (nonlinear P > 0.05) (Fig. 1). Table 2 displays the associations of PAA with SA and SI. After adjusting for various potential confounders in Model 3, we found that compared with participants in the lowest quartile of PAA, those in the highest quartile had a 21.8% (OR = 1.218, 95% CI: 1.087–1.198, P for trend < 0.001) and 12.5% (OR = 1.125, 95% CI: 1.035–1.224, P for trend < 0.001) higher likelihood of SA and SI, respectively. Furthermore, the OR for per SD increment in PAA was 1.051 (95% CI: 1.013–1.091) and 1.051 (95% CI: 1.022–1.080) for SA and SI, respectively. When dichotomizing PAA, we observed that biologically older participants had a significantly higher likelihood of experiencing SA compared to their biologically younger counterparts (OR = 1.104, 95% CI: 1.018–1.198). Similar findings were observed for SI (OR = 1.064, 95% CI: 1.003–1.129).
PAA phenotypic age acceleration, OR odds ratio, CI confidence interval. Model adjusted for age, sex, education, ethnicity, employment, Townsend Deprivation Index, smoking, alcohol consumption, physical activity, sleep duration, body mass index, hypertension, diabetes, coronary heart disease, stroke, and psychiatric disorders.
After further adjustment for childhood adversity, the associations of PAA with SA/SI were not substantially modified (Supplementary Table 6). When re-analyzed using imputed covariates, the associations remained robust (Supplementary Table 7). Sensitivity analyses without adjusting for psychiatric disorders or excluding individuals with psychiatric disorders at baseline yielded similar association patterns to the primary analyses (Supplementary Table 8). Sensitivity analyses adjusting for neurological disorders, excluding cases of self-reported suicide attempts or self-harm at baseline, categorizing PAA into quintiles, or making additional adjustments for baseline cancer did not change the robustness of the associations (Supplementary Tables 9–11). Excluding baseline depression or CVD cases did not essentially affect the associations (Supplementary Table 12).
Moderators of the associations of PAA with SA and SI
When PAA-SA associations were stratified by sociodemographic, lifestyle, and health-related factors, we did not observe significant interactions except for sex and age. In other words, the detrimental impact of biological aging on SA was more pronounced in younger (<60 years) and male participants (P for interaction <0.05) (Fig. 2). As for SI, subgroup analyses implied that SES, PA, physical disorders, and psychiatric disorders (especially depression) markedly modified the associations with biological aging (P for interaction <0.05) (Fig. 3).
TDI townsend deprivation index, BMI body mass index, CI confidence interval, OR odd ratio. Biologically younger participants were used as the reference group. aPhysical disorders referred to any of hypertension, diabetes, coronary heart disease, and stroke. bPsychiatric disorders referred to any of anxiety, depression, bipolar disorder and schizophrenia. Models adjusted for age, sex, education, ethnicity, employment, TDI, smoking, alcohol consumption, physical activity, sleep duration, BMI, physical disorders, and psychiatric disorders (excluding stratification factors). Bold suggested significant interactions.
TDI townsend deprivation index, BMI body mass index, CI confidence interval, OR odd ratio. Biologically younger participants were used as the reference group. aPhysical disorders referred to any of hypertension, diabetes, coronary heart disease, and stroke. bPsychiatric disorders referred to any of anxiety, depression, bipolar disorder and schizophrenia. Models adjusted for age, sex, education, ethnicity, employment, TDI, smoking, alcohol consumption, physical activity, sleep duration, BMI, physical disorders, and psychiatric disorders (excluding stratification factors). Bold suggested significant interactions.
Discussion
Based on a large prospective cohort, the study findings indicated that PAA-measured biological aging was significantly associated with increased odds of SA and SI independent of various confounders. Specifically, compared to their biologically younger counterparts, individuals who were biologically older at the same chronological age were more likely to experience SA/SI at follow-up. Notably, several sociodemographic factors (e.g., age, sex, and SES), lifestyle factors (e.g., PA), and health-related factors (e.g., physical and psychiatric disorders) were identified as moderators of the associations. These findings hold significant public health implications, suggesting that interventions aimed at slowing biological aging could potentially contribute to suicide prevention.
To the best of our knowledge, this is the first study to longitudinally investigate the relationship between PAA-measured biological aging and SA/SI. While no previous research has specifically examined the association between biological aging and SA/SI using the UK Biobank cohort, multiple studies using the same cohort have demonstrated significant links between PAA-quantified biological aging and other health outcomes (e.g., anxiety, depression, cancer, and neurological disorders) [16, 23, 35, 36], which are closely associated with suicide risk, potentially supporting our findings. The consistency of these findings across different outcomes using clinical markers of biological aging provided further support for the validity of our approach. Moreover, extensive prior studies have demonstrated a link between suicide and biological aging [11,12,13,14,15]. For example, Lima et al. conducted an analysis of blood DNA methylation in 144 individuals with BD and discovered that accelerated epigenetic aging was significantly more prevalent among those with a history of SA compared to those without such a history [11]. In a case-control study of 248 individuals diagnosed with BD, researchers observed that an increased frequency of SA correlated with shorter telomeres in the case group [14]. In terms of SI, a case-control study in 121 patients with schizophrenia suggested that individuals with SI exhibited significant epigenetic senescence [15]. Nonetheless, these investigations emphasized the hypothesis that SA/SI may contribute to accelerated aging. To our knowledge, only one study so far has emphasized the opposite process [21]. Similar to our findings, a case-control study by Martinez et al. in 143 individuals (60 with BD) found that telomere length was significantly associated with SI [21]. The representativeness of this study was limited by the cross-sectional design with susceptible individuals [21]. Historically, studies on this topic have relied on expensive and time-consuming molecular techniques to assess biological aging, typically focusing on mental disorder patients within small samples [11,12,13,14,15]. The present study adopted more accessible and cost-effective clinical markers as indicators of biological aging, which may significantly enhance the scope of suicide prevention strategies. The hyperactivity of the HPA axis was associated with an increased risk of suicidal behavior [17]. Meanwhile, the function of the HPA axis declined with biological aging [18]. Hence, dysregulation of HPA might be one of the neurobiological mechanisms underlying the prospective associations between biological aging and suicide-related behaviors identified in the current study. Also, systemic inflammatory dysregulation [19, 20] and stress-related hormonal disturbances [21, 22] might act as underlying mechanisms of the associations. Furthermore, mounting studies have established that PAA-measured biological aging is a valid predictor of a range of physical and psychological disorders that have been documented to be significant risk factors for SA/SI [1, 10, 16, 23]. Such the above-mentioned evidence supported the study findings that biological aging might be associated with the risk of SA and SI. The findings of this study underscored the importance of considering biological aging in suicide research. Biological aging based on clinical markers integrates multiple physiological pathways and could contribute to an improved understanding of the mechanisms underlying suicidal behaviors. This shift in focus might open new avenues for suicide intervention, for instance, strategies aimed at slowing biological aging (e.g., lifestyle modifications) could complement existing psychological or social interventions.
Moreover, we observed that the detrimental impact of PAA-measured biological aging on SA was more pronounced in younger and male participants. The aging process may lead to diverse perceptions of life expectancy and quality of life, where younger individuals often face significant challenges in accepting the inherent limitations of biological aging. In alignment with previous research, Gao et al. found that males were at higher risk for anxiety/depression associated with biological aging [16]. One plausible reason is that males are more associated with unhealthy lifestyle choices, such as smoking and drinking alcohol, which are significant risk factors for SA [37]. Regarding SI, we found a significant interaction term between biological aging and SES (education and employment), such that the associations between biological aging and SI were stronger among those with low SES compared to their counterparts with high SES. Prior studies have demonstrated that low-SES individuals exhibited higher SI severity and were more likely to be on a high persistent SI trajectory at follow-up [38, 39]. One possible explanation is that high-SES individuals typically possess better mental health services and more extensive social support, which may be beneficial in reducing the odds of SI [39]. Hence, it is feasible that the high SES could mitigate the adverse effects of biological aging. Moreover, previous studies have illustrated the benefits of lifestyle interventions in delaying biological aging or its health detriments. For instance, a prospective cohort study of 281,889 individuals reported that healthier lifestyles were more likely to alleviate the detrimental impact of biological aging on cancer [40]. A randomized controlled trial reported lifestyle intervention could reverse epigenetic aging [41]. Supporting these studies, the present study detected that maintaining healthy lifestyles might mitigate the harmful effects of biological aging on suicide to some extent, although significant interactions were observed only in PA. As expected, we found that the association between biological aging and SI was more pronounced in participants with physical and psychiatric disorders. Aging individuals may contemplate death more frequently as the quality of life declines, especially if suffering from long-lasting chronic diseases, which may increase the risk of SI [42]. Further research is warranted to confirm and decipher these moderators of the biological aging-suicide associations found in the study.
In addition to the prospective design with large samples, the study demonstrated for the first time that PAA-measured biological aging might be associated with SA and SI. Compared to other molecular-level indicators, clinical marker-based indicators of biological aging are more accessible and hold great promise for suicide prevention. Acknowledging several limitations of this study was warranted. First, the nature of observational studies made it impossible to rule out the possibility of unmeasured or residual confounders and to establish the causality of observed associations. Further studies, such as Mendelian randomization or experimental studies, are warranted to determine causality. Second, PhenoAge is dynamic and changes over time, but our study was limited to a single measurement and did not account for this variability. It is necessary to use repeated-measures data from multiple time points to explore how changes in PhenoAge over time affect suicide risk. Third, limitations in data availability prevented us from excluding individuals with a history of suicide at baseline, so the possibility of reverse causation remained, despite our efforts to control for a range of confounders significantly associated with suicide including common mental disorders and childhood adversity. Fourth, the definition of SA and SI in the study was based on participants’ self-reports rather than clinical diagnoses, and therefore the findings may be susceptible to recall bias. However, the measurement of suicidal behaviors used in this study has been validated by previous studies [27, 28]. Fifth, the vast majority of our study sample was white (97.4%), which limited the extrapolation of the findings. Therefore, duplicating our findings across racially diverse populations was warranted. Sixth, suicidal behaviors are the result of the interaction between genetic and environmental factors, but this study did not consider genetic information. Future research will necessitate the inclusion of genetic factors for a more comprehensive analysis. Finally, participants in the UK Biobank are typically healthier than the general population, which may have affected the representativeness of the study findings, although the external validity of the risk factor associations observed in this cohort has been previously established [43].
In summary, the large population-based prospective cohort study observed significant positive associations between PAA-quantified biological aging and SA/SI, particularly among males, young adults, individuals with low SES, and those with chronic diseases. Maintaining healthy lifestyles, such as engaging in regular PA, might somewhat modify the associations.
Data availability
Data utilized in this study are available from the UK Biobank by submitting a data request proposal (https://www.ukbiobank.ac.uk).
Code availability
Codes are available from the corresponding author upon reasonable request.
References
Fleischmann A, Paul E, Kestel D, Cao B, Ho J, Mahanani WR. Suicide worldwide in 2019. Geneva: World Health Organization. 2021.
World Health Organization. Preventing suicide: a global imperative. Geneva: World Health Organization. 2014.
Turecki G, Brent DA. Suicide and suicidal behaviour. Lancet. 2016;387:1227–39.
Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;21:29–36.
Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10:573–91.
Liu Z, Kuo PL, Horvath S, Crimmins E, Ferrucci L, Levine M. A new aging measure captures morbidity and mortality risk across diverse subpopulations from NHANES IV: a cohort study. PLoS Med. 2018;15:e1002718.
Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68:667–74.
Sanders JL, Newman AB. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol Rev. 2013;35:112–31.
Kuo CL, Pilling LC, Liu Z, Atkins JL, Levine ME. Genetic associations for two biological age measures point to distinct aging phenotypes. Aging Cell. 2021;20:e13376.
Wang T, Duan W, Jia X, Huang X, Liu Y, Meng F, et al. Associations of combined phenotypic ageing and genetic risk with incidence of chronic respiratory diseases in the UK Biobank: a prospective cohort study. Eur Respir J. 2024;63:2301720.
Lima CNC, Kovács EHC, Mirza S, Del Favero-Campbell A, Diaz AP, Quevedo J, et al. Association between the epigenetic lifespan predictor GrimAge and history of suicide attempt in bipolar disorder. Neuropsychopharmacology. 2023;48:954–62.
Jeremian R, Bani-Fatemi A, Strauss JS, Tasmim S, Dada O, Graff-Guerrero A, et al. Investigation of accelerated epigenetic aging in individuals suffering from schizophrenia in the context of lifetime suicide attempt. Schizophr Res. 2022;243:222–4.
Ochi S, Roy B, Prall K, Shelton RC, Dwivedi Y. Strong associations of telomere length and mitochondrial copy number with suicidality and abuse history in adolescent depressed individuals. Mol Psychiatry. 2023;28:3920–9.
Birkenæs V, Elvsåshagen T, Westlye LT, Høegh MC, Haram M, Werner MCF, et al. Telomeres are shorter and associated with number of suicide attempts in affective disorders. J Affect Disord. 2021;295:1032–9.
Dada O, Jeremian R, Dai N, Strauss J, Zai C, Graff A, et al. Investigation of accelerated epigenetic aging in individuals experiencing suicidal ideation. Schizophr Res. 2022;243:307–9.
Gao X, Geng T, Jiang M, Huang N, Zheng Y, Belsky DW, et al. Accelerated biological aging and risk of depression and anxiety: evidence from 424,299 UK Biobank participants. Nat Commun. 2023;14:2277.
Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. Am J Psychiatry. 2001;158:748–53.
Gaffey AE, Bergeman CS, Clark LA, Wirth MM. Aging and the HPA axis: stress and resilience in older adults. Neurosci Biobehav Rev. 2016;68:928–45.
Xiao C, Beitler JJ, Peng G, Levine ME, Conneely KN, Zhao H, et al. Epigenetic age acceleration, fatigue, and inflammation in patients undergoing radiation therapy for head and neck cancer: a longitudinal study. Cancer. 2021;127:3361–71.
Beurel E, Jope RS. Inflammation and lithium: clues to mechanisms contributing to suicide-linked traits. Transl Psychiatry. 2014;4:e488.
Martinez D, Lavebratt C, Millischer V, de Jesus RDPV, Pires T, Michelon L, et al. Shorter telomere length and suicidal ideation in familial bipolar disorder. PLoS One. 2022;17:e0275999.
Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, et al. Molecular signatures of major depression. Curr Biol. 2015;25:1146–56.
Ma Z, Zhu C, Wang H, Ji M, Huang Y, Wei X, et al. Association between biological aging and lung cancer risk: cohort study and Mendelian randomization analysis. iScience. 2023;26:106018.
Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779.
Elliott P, Peakman TC. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37:234–44.
Davis KAS, Coleman JRI, Adams M, Allen N, Breen G, Cullen B, et al. Mental health in UK Biobank - development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open. 2020;6:e18.
Harrison R, Munafò MR, Davey Smith G, Wootton RE. Examining the effect of smoking on suicidal ideation and attempts: triangulation of epidemiological approaches. Br J Psychiatry. 2020;217:701–7.
Edwards AC, Gentry AE, Peterson RE, Webb BT, Mościcki EK. Multifaceted risk for non-suicidal self-injury only versus suicide attempt in a population-based cohort of adults. J Affect Disord. 2023;333:474–81.
Foster HME, Celis-Morales CA, Nicholl BI, Petermann-Rocha F, Pell JP, Gill JMR, et al. The effect of socioeconomic deprivation on the association between an extended measurement of unhealthy lifestyle factors and health outcomes: a prospective analysis of the UK Biobank cohort. Lancet Public Health. 2018;3:e576–85.
Geng T, Zhu K, Lu Q, Wan Z, Chen X, Liu L, et al. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: A cohort study. PLoS Med. 2023;20:e1004135.
Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank study. JAMA Cardiol. 2018;3:693–702.
Huang S, Zhong D, Lv Z, Cheng J, Zou X, Wang T, et al. Associations of multiple plasma metals with the risk of metabolic syndrome: A cross-sectional study in the mid-aged and older population of China. Ecotoxicol Environ Saf. 2022;231:113183.
Yang G, Cao X, Li X, Zhang J, Ma C, Zhang N, et al. Association of unhealthy lifestyle and childhood adversity with acceleration of aging among UK Biobank participants. JAMA Netw Open. 2022;5:e2230690.
Barboza-Salerno GE, Meshelemiah JCA. Associations between early child adversity and lifetime suicide attempts among gender diverse individuals: a moderated mediation. Child Abuse Negl. 2024;149:106705.
Mak JKL, McMurran CE, Kuja-Halkola R, Hall P, Czene K, Jylhävä J, et al. Clinical biomarker-based biological aging and risk of cancer in the UK Biobank. Br J Cancer. 2023;129:94–103.
Mak JKL, McMurran CE, Hägg S. Clinical biomarker-based biological ageing and future risk of neurological disorders in the UK Biobank. J Neurol Neurosurg Psychiatry. 2024;95:481–4.
Kim H, Ryu S, Jeon HJ, Roh S. Lifestyle factors and suicide risk: a nationwide population-based study. J Affect Disord. 2023;328:215–21.
Cambron C, Jaggers JW. Examining area- and individual-level differences in suicide ideation severity and suicide attempt among youth. J Res Adolesc. 2024;34:35–44.
Choi M, Lee EH, Sempungu JK, Lee YH. Long-term trajectories of suicide ideation and its socioeconomic predictors: A longitudinal 8-year follow-up study. Soc Sci Med. 2023;326:115926.
Wang X, Peng Y, Liu F, Wang P, Si C, Gong J, et al. Joint association of biological aging and lifestyle with risks of cancer incidence and mortality: a cohort study in the UK Biobank. Prev Med. 2024;182:107928.
Fitzgerald KN, Hodges R, Hanes D, Stack E, Cheishvili D, Szyf M, et al. Potential reversal of epigenetic age using a diet and lifestyle intervention: a pilot randomized clinical trial. Aging. 2021;13:9419–32.
Beghi M, Butera E, Cerri CG, Cornaggia CM, Febbo F, Mollica A, et al. Suicidal behaviour in older age: a systematic review of risk factors associated to suicide attempts and completed suicides. Neurosci Biobehav Rev. 2021;127:193–211.
Batty GD, Gale CR, Kivimäki M, Deary IJ, Bell S. Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. BMJ. 2020;368:m131.
Acknowledgements
The authors gratefully thank all the participants and professionals contributing to the UK Biobank. The current study was done using the UK Biobank resource under application 91536.
Funding
This research was supported by Shandong Provincial Natural Science Foundation [grant number: ZR2021QH310] and National Natural Science Foundation of China [grant number: 82473710].
Author information
Authors and Affiliations
Contributions
WH, BL, and CJ conceived and designed the study. BL and CJ prepared the data. WH conducted the data analysis, performed interpretation of the results and drafted the paper. GT, ZS, BL and CJ made critical revisions. All authors agreed on the final version of the paper and take responsibility for its content. Additional contributions: we gratefully thank all the participants and professionals contributing to the UK Biobank.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The Northwest Multi-Center Research Ethics Committee provided ethical approval for the UK Biobank project. All participants gave informed consent before being recruited. The current study was conducted using the UK Biobank resource under Application Number 91536. The study complied with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Hu, W., Shen, Z., Tian, G. et al. Associations of clinical biomarker-based biological aging with suicide attempts and suicidal ideation: evidence from 124,529 UK Biobank participants. Transl Psychiatry 15, 204 (2025). https://doi.org/10.1038/s41398-025-03412-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-025-03412-5