Abstract
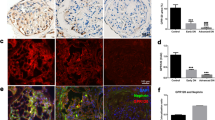
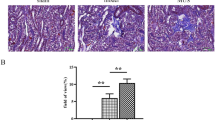
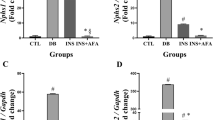
Epalrestat is an inhibitor of aldose reductase in the polyol pathway and is used for the management of diabetic neuropathy clinically. Our pilot experiments and accumulated evidences showed that epalrestat inhibited polyol pathway and reduced sorbitol production, and suggested the potential renal protection effects of epalrestat on diabetic nephropathy (DN). To evaluate the protective effect of epalrestat, the db/db mice were used and exposed to epalrestat for 8 weeks, both the physiopathological condition and function of kidney were examined. For the first time, we showed that epalrestat markedly reduced albuminuria and alleviated the podocyte foot process fusion and interstitial fibrosis of db/db mice. Metabolomics was employed, and metabolites in the plasma, renal cortex, and urine were profiled using a gas chromatography-mass spectrometry (GC/MS)-based metabolomic platform. We observed an elevation of sorbitol and fructose, and a decrease of myo-inositol in the renal cortex of db/db mice. Epalrestat reversed the renal accumulation of the polyol pathway metabolites of sorbitol and fructose, and increased myo-inositol level. Moreover, the upregulation of aldose reductase, fibronectin, collagen III, and TGF-β1 in renal cortex of db/db mice was downregulated by epalrestat. The data suggested that epalrestat has protective effects on DN, and the inhibition of aldose reductase and the modulation of polyol pathway in nephritic cells be a potentially therapeutic strategy for DN.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Oates PJ. Aldose reductase inhibitors and diabetic kidney disease. Curr Opin Investig Drugs. 2010;11:402–17.
Nishikawa T, Edelstein D, Brownlee M. The missing link: a single unifying mechanism for diabetic complications. Kidney Int Suppl. 2000;77:S26–30.
Hers HG. Le mécanisme de la transformation de glucose en fructose par les vésicules séminales. Biochim Biophys Acta. 1956;22:202–3.
Dunlop M. Aldose reductase and the role of the polyol pathway in diabetic nephropathy. Kidney Int Suppl. 2000;77:S3–12.
Yabe-Nishimura C. Aldose reductase in glucose toxicity: a potential target for the prevention of diabetic complications. Pharmacol Rev. 1998;50:21–34.
Van Heyningen R. Formation of polyols by the lens of the rat with ‘sugar’ cataract. Nature. 1959;184:194–5.
Greene DA, Chakrabarti S, Lattimer SA, Sima AA. Role of sorbitol accumulation and myo-inositol depletion in paranodal swelling of large myelinated nerve fibers in the insulin-deficient spontaneously diabetic bio-breeding rat. Reversal by insulin replacement, an aldose reductase inhibitor, and myo-inositol. J Clin Invest. 1987;79:1479–85.
Greene DA, Lattimer SA, Sima AA. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987;316:599–606.
Tang W, Martin K, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87.
Huang Z, Hong Q, Zhang X, Xiao W, Wang L, Cui S, et al. Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun Signal. 2017;15:3.
Ramirez MA, Borja NL. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008;28:646–55.
Tsugawa T, Shinohara R, Nagasaka A, Nakano I, Takeda F, Nagata M, et al. Alteration of urinary sorbitol excretion in WBN-kob diabetic rats - treatment with an aldose reductase inhibitor. J Endocrinol. 2004;181:429–35.
Itagaki I, Shimizu K, Kamanaka Y, Ebata K, Kikkawa R, Haneda M, et al. The effect of an aldose reductase inhibitor (Epalrestat) on diabetic nephropathy in rats. Diabetes Res Clin Pract. 1994;25:147–54.
Li M, Wang X, Aa J, Qin W, Zha W, Ge Y, et al. GC/TOFMS analysis of metabolites in serum and urine reveals metabolic perturbation of TCA cycle in db/db mice involved in diabetic nephropathy. Am J Physiol Ren Physiol. 2013;304:F1317–24.
Chang HH, Chao HN, Walker CS, Choong SY, Phillips A, Loomes KM. Renal depletion of myo-inositol is associated with its increased degradation in animal models of metabolic disease. Am J Physiol Ren Physiol. 2015;309:F755–63.
Fonteles MC. Myo-inositol concentration is strongly reduced in the renal cortex of STZ-diabetic rats. FASEB J. 2017;31:673–4.
Hotta N, Kawamori R, Fukuda M, Shigeta Y. The Aldose Reductase Inhibitor–Diabetes Complications Trial Study G. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012;29:1529–33.
Melhem MF, Craven PA, Liachenko J, DeRubertis FR. α-Lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J Am Soc Nephrol. 2002;13:108–16.
Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, et al. IL-10/TGF-β–modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–42.
Pei F, Li BY, Zhang Z, Yu F, Li XL, Lu WD, et al. Beneficial effects of phlorizin on diabetic nephropathy in diabetic db/db mice. J Diabetes Complications. 2014;28:596–603.
Liu J, Chen Z, Zhang Y, Zhang M, Zhu X, Fan Y, et al. Rhein protects pancreatic β-cells from dynamin-related protein-1–mediated mitochondrial fission and cell apoptosis under hyperglycemia. Diabetes. 2013;62:3927–35.
Mac-Moune Lai F, Szeto CC, Choi PC, Ho KK, Tang NL, Chow KM, et al. Isolate diffuse thickening of glomerular capillary basement membrane: a renal lesion in prediabetes? Mod Pathol. 2004;17:1506–12.
van den berg JG, van den Bergh Weerman MA, Assmann KJM, Weening JJ, Florquin S. Podocyte foot process effacement is not correlated with the level of proteinuria in human glomerulopathies. Kidney Int. 2004;66:1901–6.
Guo J, Yong Y, Aa J, Cao B, Sun R, Yu X, et al. Compound danshen dripping pills modulate the perturbed energy metabolism in a rat model of acute myocardial ischemia. Sci Rep. 2016;6:37919.
Warrack BM, Hnatyshyn S, Ott KH, Reily MD, Sanders M, Zhang H, et al. Normalization strategies for metabonomic analysis of urine samples. J Chromatogr B Biomed Appl. 2009;877:547–52.
He J, Zhu Y, Aa J, Smith PF, De Ridder D, Wang G, et al. Brain metabolic changes in rats following acoustic trauma. Front Neurosci. 2017;11:148.
Manly BFJ, Alberto JAN. Multivariate statistical methods. A primer. 4th edn. (Chapman & Hall/CRC, New York, United States, 2016).
Tang L, Peng S, Bi Y, Shan P, Hu X. A new method combining LDA and PLS for dimension reduction. PLoS ONE. 2014;9:e96944.
Wiklund S, Johansson E, Sjöström L, Mellerowicz EJ, Edlund U, Shockcor JP, et al. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal Chem. 2008;80:115–22.
Sedghipour MR, Sadeghi-Bazargani H. Applicability of supervised discriminant analysis models to analyze astigmatism clinical trial data. Clin Ophthalmol. 2012;6:1499–506.
Worley B, Powers R. Multivariate analysis in metabolomics. Curr Metab. 2013;1:92–107.
Sango K, Suzuki T, Yanagisawa H, Takaku S, Hirooka H, Tamura M, et al. High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. J Neurochem. 2006;98:446–58.
Suzuki T, Mizuno K, Yashima S, Watanabe K, Taniko K, Yabe-Nishimura C, et al. Characterization of polyol pathway in schwann cells isolated from adult rat sciatic nerves. J Neurosci Res. 1999;57:495–503.
Ferraz M, Ishii-Iwamoto EL, Batista MR, Brunaldi K, Bazotte RB. Sorbitol accumulation in rats kept on diabetic condition for short and prolonged periods. Acta Pharmacol Sin. 1997;18:309–11.
Kinoshita JH. Cataracts in galactosemia. The Jonas S. Friedenwald Memorial Lecture. Invest Ophthalmol. 1965;4:786–99.
Kinoshita JH. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974;13:713–24.
Cammarata PR, Chen HQ, Yang J, Yorio T. Modulation of myo-[3H]inositol uptake by glucose and sorbitol in cultured bovine lens epithelial cells. II. Characterization of high- and low-affinity myo-inositol transport sites. Invest Ophthalmol Vis Sci. 1992;33:3572–80.
Haneda M, Kikkawa R, Arimura T, Ebata K, Togawa M, Maeda S, et al. Glucose inhibits myo-inositol uptake and reduces myo-inositol content in cultured rat glomerular mesangial cells. Metabolism. 1990;39:40–45.
Raccah D, Coste T, Cameron NE, Dufayet D, Vague P, Hohman TC, et al. Effect of the aldose reductase inhibitor tolrestat on nerve conduction velocity, NA/K ATPase activity, and polyols in red blood cells, sciatic nerve, kidney cortex, and kidney medulla of diabetic rats. J Diabetes Complications. 1998;12:154–62.
Croze ML, Soulage CO. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 2013;95:1811–27.
Kinoshita JH, Nishimura C. The involvement of aldose reductase in diabetic complications. Diabetes Metab Rev. 1988;4:323–37.
Larkins RG, Dunlop ME. The link between hyperglycaemia and diabetic nephropathy. Diabetologia. 1992;35:499–504.
Yin XX, Zhang YD, Shen JP, Wu HW, Zhu X, Li LM, et al. Protective effects of bendazac lysine on early experimental diabetic nephropathy in rats. Acta Pharmacol Sin. 2005;26:721–8.
Kasajima H, Yamagishi SI, Sugai S, Yagihashi N, Yagihashi S. Enhanced in situ expression of aldose reductase in peripheral nerve and renal glomeruli in diabetic patients. J Peripher Nerv Syst. 2002;7:134.
Corder CN, Braughler JM, Culp PA. Quantitative histochemistry of the sorbitol pathway in glomeruli and small arteries of human diabetic kidney. Folia Histochem Cytochem (Krakow). 1979;17:137–45.
Sands JM, Terada Y, Bernard LM, Knepper MA. Aldose reductase activities in microdissected rat renal tubule segments. Am J Physiol. 1989;256:F563–69.
Bondy CA, Lightman SL, Lightman SL. Developmental and physiological regulation of aldose reductase mRNA expression in renal medulla. Mol Endocrinol. 1989;3:1409–16.
Lorenzi M. The polyol pathway as a mechanism for diabetic retinopathy: attractive, elusive, and resilient. Exp Diabetes Res. 2007;2007:61038.
Hashimoto Y, Yamagishi S, Mizukami H, Yabe-Nishimura C, Lim SW, Kwon HM, et al. Polyol pathway and diabetic nephropathy revisited: Early tubular cell changes and glomerulopathy in diabetic mice overexpressing human aldose reductase. J Diabetes Investig. 2011;2:111–22.
Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
Nakayama T, Kosugi T, Gersch M, Connor T, Sanchez-Lozada LG, Lanaspa MA, et al. Dietary fructose causes tubulointerstitial injury in the normal rat kidney. Am J Physiol Ren Physiol. 2010;298:F712–20.
Lanaspa MA, Ishimoto T, Cicerchi C, Tamura Y, Roncal-Jimenez CA, Chen W, et al. Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J Am Soc Nephrol. 2014;25:2526–38.
Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, et al. Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Ren Physiol. 2007;293:F1256–61.
Chalk C, Benstead TJ, Moore F. Aldose reductase inhibitors for the treatment of diabetic polyneuropathy. Cochrane Database Syst Rev 2007;1:CD004572.
Wei J, Zhang Y, Luo Y, Wang Z, Bi S, Song D, et al. Aldose reductase regulates miR-200a-3p/141-3p to coordinate Keap1–Nrf2, Tgfβ1/2, and Zeb1/2 signaling in renal mesangial cells and the renal cortex of diabetic mice. Free Radic Biol Med. 2014;67:91–102.
Iso K, Tada H, Kuboki K, Inokuchi T. Long-term effect of epalrestat, an aldose reductase inhibitor, on the development of incipient diabetic nephropathy in Type 2 diabetic patients. J Diabetes Complications. 2001;15:241–4.
Zhang ZH, Wei F, Vaziri ND, Cheng XL, Bai X, Lin RC, et al. Metabolomics insights into chronic kidney disease and modulatory effect of rhubarb against tubulointerstitial fibrosis. Sci Rep. 2015;5:14472.
Newgard Christopher B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012;15:606–14.
Kumar MA, Bitla ARR, Raju KVN, Manohar SM, Kumar VS, Narasimha SRPVL. Branched chain amino acid profile in early chronic kidney disease. Saudi J Kidney Dis Transpl. 2012;23:1202–7.
Cano NJM, Fouque D, Leverve XM. Application of branched-chain amino acids in human pathological states: renal failure. J Nutr. 2006;136:299S–307S.
Mi N, et al. Branched-chain amino acids attenuate early kidney injury in diabetic rats. Biochem Biophys Res Commun. 2015;466:240–6.
Men LH, Pi ZF, Zhou Y, Liu YY, Wei MY, Song FR, et al. Metabolomics insights into diabetes nephropathy and protective effects of Radix Scutellariae on rats using ultra-high performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. RSC Adv. 2017;7:16494–504.
Wei T, Zhao L, Jia J, Xia H, Du Y, Lin Q, et al. Metabonomic analysis of potential biomarkers and drug targets involved in diabetic nephropathy mice. Sci Rep. 2015;5:11998.
Kopple JD. Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr. 2007;137:1586S–1590SS. discussion 97S–98S.
Itoh Y, Ezawa A, Kikuchi K, Tsuruta Y, Niwa T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal Bioanal Chem. 2012;403:1841–50.
Macdonald G, Assef R, Guiffre A, Lo E. Vasoconstrictor effects of uridine and its nucleotides and their inhibition by adenosine. Clin Exp Pharmacol Physiol. 1984;11:381–4.
Liu J, Wang C, Liu F, Lu Y, Cheng J. Metabonomics revealed xanthine oxidase-induced oxidative stress and inflammation in the pathogenesis of diabetic nephropathy. Anal Bioanal Chem. 2015;407:2569–79.
Acknowledgements
We want to thank Dr. Xiao-dong Zhu for his help in technical support for transmission electron microscopy study in the Research Institute of Nephrology of PLA, Jinling Hospital, Nanjing, China. This study was financially supported by the National Natural Science Foundation of the People’s Republic of China (81530098), the National Key Special Project of Science and Technology for Innovation Drugs of China (2017ZX09301013, 2015zx09501001), the Natural Science Foundation of Jiangsu Province (BL2014070), and the project of university collaborative innovation center of Jiangsu province (Modern Chinese Medicine Center and Biological Medicine Center).
Author contributions
JH, H-xG, NY, X-dZ, C-hZ, and J-kW performed the experiments; JH and R-bS analyzed the data; JH and J-yA interpreted the results of the experiment, prepared figures, and drafted the manuscript; YX, J-wZ, and FD helped to design the research; J-yA and G-jW revised the manuscript; G-jW approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
He, J., Gao, Hx., Yang, N. et al. The aldose reductase inhibitor epalrestat exerts nephritic protection on diabetic nephropathy in db/db mice through metabolic modulation. Acta Pharmacol Sin 40, 86–97 (2019). https://doi.org/10.1038/s41401-018-0043-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-018-0043-5
Keywords
This article is cited by
-
Repurposing of epalrestat for neuroprotection in parkinson’s disease via activation of the KEAP1/Nrf2 pathway
Journal of Neuroinflammation (2025)
-
Inhibitory Impact of Quercetin Nanoparticles on Polyol Pathway in Hyperthyroidism Rats
BioNanoScience (2024)
-
Novel role for epalrestat: protecting against NLRP3 inflammasome-driven NASH by targeting aldose reductase
Journal of Translational Medicine (2023)
-
Astragalin ameliorates renal injury in diabetic mice by modulating mitochondrial quality control via AMPK-dependent PGC1α pathway
Acta Pharmacologica Sinica (2023)
-
2′-Hydroxy-4′,5′-dimethoxyacetophenone Exhibit Collagenase, Aldose Reductase Inhibition, and Anticancer Activity Against Human Leukemic Cells: An In Vitro, and In Silico Study
Molecular Biotechnology (2023)