Abstract
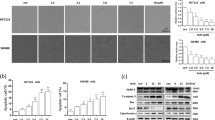
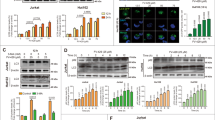
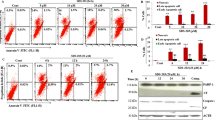
The mechanism underlying the resistance of cancer cells to chemotherapeutic drug varies with different cancer cells. Recent evidence shows that lysosomal function is associated with drug resistance of cancer cells. Artesunate, a derivative of artemisinin, displays broad antitumor activity and direct cytotoxicity on various tumor cells. Our previous study shows that artesunate increases autophagosome accumulation, while significantly decreases autolysosome number in cancer cells, suggesting that artesunate might impair the lysosomal function. In this study, we investigated the effects of artesunate on lysosomal function and its relationship with chemotherapeutic drug resistance in cancer cells. We found that the lysosomal function was significantly enhanced in two drug-resistant (A549/TAX and A549/DDP) cells. Furthermore, we showed that the enhanced lysosomal function by overexpression of transcription factor EB (TFEB) significantly increased MCF-7 cells resistance to doxorubicin (DOX), whereas the decreased lysosomal function by TFEB-knockdown or lysosome inhibitor chloroquine increased MCF-7 cells sensitivity to DOX. Treatment of A549/TAX cells with artesunate (2.5–50 μM) dose-dependently inhibited lysosomal function and the clearance of dysfunctional mitochondria, and induced cell apoptosis. Moreover, we demonstrated that artesunate exerted more potent inhibition on the resistant (A549/TAX and MCF-7/ADR) cells with higher activity of lysosomal function. Our results suggest that artesunate or other inhibitors of lysosomal function would be potential in the treatment of cancer cells with drug resistance caused by the enhanced lysosomal function.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–54.
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers. 2014;6:1769–92.
Shiraishi N, Akiyama S, Kobayashi M, Kuwano M. Lysosomotropic agents reverse multiple drug resistance in human cancer cells. Cancer Lett. 1986;30:251–9.
Rios-Luci C, Garcia-Alonso S, Diaz-Rodriguez E, Nadal-Serrano M, Arribas J, Ocana A, et al. Resistance to the antibody-drug conjugate T-DM1 is based in a reduction in lysosomal proteolytic activity. Cancer Res. 2017;77:4639–51.
Appelqvist H, Waster P, Kagedal K, Ollinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5:214–26.
de Duve C. The lysosome turns fifty. Nat Cell Biol. 2005;7:847–9.
Ohkuma S, Moriyama Y, Takano T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc Natl Acad Sci USA. 1982;79:2758–62.
Bucci C, Thomsen P, Nicoziani P, McCarthy J, van Deurs B. Rab7: a key to lysosome biogenesis. Mol Biol Cell. 2000;11:467–80.
Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47.
Boya P. Lysosomal function and dysfunction: mechanism and disease. Antioxid Redox Signal. 2012;17:766–74.
Kallifatidis G, Hoepfner D, Jaeg T, Guzman EA, Wright AE. The marine natural product manzamine A targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells. Mar Drugs. 2013;11:3500–16.
Zhitomirsky B, Assaraf YG. Lysosomal sequestration of hydrophobic weak base chemotherapeutics triggers lysosomal biogenesis and lysosome-dependent cancer multidrug resistance. Oncotarget. 2015;6:1143–56.
Kumar B, Kalvala A, Chu S, Rosen S, Forman SJ, Marcucci G, et al. Antileukemic activity and cellular effects of the antimalarial agent artesunate in acute myeloid leukemia. Leuk Res. 2017;59:124–35.
Vandewynckel YP, Laukens D, Geerts A, Vanhove C, Descamps B, Colle I, et al. Therapeutic effects of artesunate in hepatocellular carcinoma: repurposing an ancient antimalarial agent. Eur J Gastroenterol Hepatol. 2014;26:861–70.
Augustin Y, Krishna S, Kumar D, Pantziarka P. The wisdom of crowds and the repurposing of artesunate as an anticancer drug. Ecancermedicalscience. 2015;9:ed50.
Zhou C, Pan W, Wang XP, Chen TS. Artesunate induces apoptosis via a Bak-mediated caspase-independent intrinsic pathway in human lung adenocarcinoma cells. J Cell Physiol. 2012;227:3778–86.
Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol Cancer Ther. 2011;10:2224–33.
Beccafico S, Morozzi G, Marchetti MC, Riccardi C, Sidoni A, Donato R, et al. Artesunate induces ROS- and p38 MAPK-mediated apoptosis and counteracts tumor growth in vivo in embryonal rhabdomyosarcoma cells. Carcinogenesis. 2015;36:1071–83.
Button RW, Lin F, Ercolano E, Vincent JH, Hu B, Hanemann CO, et al. Artesunate induces necrotic cell death in schwannoma cells. Cell Death Dis. 2014;5:e1466.
Marwaha R, Sharma M, DQ-Red BSA. Trafficking assay in cultured cells to assess cargo delivery to lysosomes. Bio Protoc. 2017;7:e2571.
Greenshields AL, Fernando W, Hoskin DW. The anti-malarial drug artesunate causes cell cycle arrest and apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3 breast cancer cells. Exp Mol Pathol. 2019;107:10–22.
Jing W, Dong H, Min M, Runpeng Z, Xuewei X, Ru C, et al. Dependence of artesunate on long noncoding RNA-RP11 to inhibit epithelial-mesenchymal transition of hepatocellular carcinoma. J Cell Biochem. 2019;120:6026–34.
DeVorkin L, Gorski SM. LysoTracker staining to aid in monitoring autophagy in Drosophila. Cold Spring Harb Protoc. 2014;2014:951–8.
Button RW, Roberts SL, Willis TL, Hanemann CO, Luo S. Accumulation of autophagosomes confers cytotoxicity. J Biol Chem. 2017;292:13599–614.
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, et al. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14:1435–55.
Piao S, Amaravadi RK. Targeting the lysosome in cancer. Ann N Y Acad Sci. 2016;1371:45–54.
Kallunki T, Olsen OD, Jaattela M. Cancer-associated lysosomal changes: friends or foes? Oncogene. 2013;32:1995–2004.
Fehrenbacher N, Gyrd-Hansen M, Poulsen B, Felbor U, Kallunki T, Boes M, et al. Sensitization to the lysosomal cell death pathway upon immortalization and transformation. Cancer Res. 2004;64:5301–10.
Rochefort H, Garcia M, Glondu M, Laurent V, Liaudet E, Rey JM, et al. Cathepsin D in breast cancer: mechanisms and clinical applications, a 1999 overview. Clin Chim Acta. 2000;291:157–70.
Winiarski BK, Wolanska KI, Rai S, Ahmed T, Acheson N, Gutowski NJ, et al. Epithelial ovarian cancer-induced angiogenic phenotype of human omental microvascular endothelial cells may occur independently of VEGF signaling. Transl Oncol. 2013;6:703–14.
Vetvicka V, Vetvickova J, Benes P. Role of enzymatically inactive procathepsin D in lung cancer. Anticancer Res. 2004;24:2739–43.
Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–52.
Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011;21:228–37.
Fei Y, Xiong Y, Shen X, Zhao Y, Zhu Y, Wang L, et al. Cathepsin L promotes ionizing radiation-induced U251 glioma cell migration and invasion through regulating the GSK-3beta/CUX1 pathway. Cell Signal. 2018;44:62–71.
Foghsgaard L, Wissing D, Mauch D, Lademann U, Bastholm L, Boes M, et al. Cathepsin B acts as a dominant execution protease in tumor cell apoptosis induced by tumor necrosis factor. J Cell Biol. 2001;153:999–1010.
Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Investig. 2000;106:1127–37.
Leist M, Jaattela M. Four deaths and a funeral: from caspases to alternative mechanisms. Nat Rev Mol Cell Biol. 2001;2:589–98.
Serrano-Puebla A, Boya P. Lysosomal membrane permeabilization in cell death: new evidence and implications for health and disease. Ann N Y Acad Sci. 2016;1371:30–44.
Messner B, Ploner C, Laufer G, Bernhard D. Cadmium activates a programmed, lysosomal membrane permeabilization-dependent necrosis pathway. Toxicol Lett. 2012;212:268–75.
Ou J, Peng Y, Yang W, Zhang Y, Hao J, Li F, et al. ABHD5 blunts the sensitivity of colorectal cancer to fluorouracil via promoting autophagic uracil yield. Nat Commun. 2019;10:1078.
Bao J, Zheng L, Zhang Q, Li X, Zhang X, Li Z, et al. Deacetylation of TFEB promotes fibrillar Abeta degradation by upregulating lysosomal biogenesis in microglia. Protein Cell. 2016;7:417–33.
Yang ND, Tan SH, Ng S, Shi Y, Zhou J, Tan KS, et al. Artesunate induces cell death in human cancer cells via enhancing lysosomal function and lysosomal degradation of ferritin. J Biol Chem. 2014;289:33425–41.
Skulachev VP. Role of uncoupled and non-coupled oxidations in maintenance of safely low levels of oxygen and its one-electron reductants. Q Rev Biophys. 1996;29:169–202.
Lenaz G. Mitochondria and reactive oxygen species. Which role in physiology and pathology? Adv Exp Med Biol. 2012;942:93–136.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of Jiangsu Province (No. BK20151233), the Scientific and Technological Innovation Team Building Program of Suzhou Vocational Health College (SZWZYTD201801), the Youth Project of Health and Family Planning Bureau of Suzhou New District (No. 2017Q005), Suzhou industrial technology innovation (No. SYSD2016062), the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD).
Author information
Authors and Affiliations
Contributions
ZL completed most of the research work; YTZ and MX performed some research work and wrote the paper. JLQ provided suggestions for the research. SQL and FL designed the research work and revised the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Z., Zhu, Yt., Xiang, M. et al. Enhanced lysosomal function is critical for paclitaxel resistance in cancer cells: reversed by artesunate. Acta Pharmacol Sin 42, 624–632 (2021). https://doi.org/10.1038/s41401-020-0445-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-020-0445-z
Keywords
This article is cited by
-
Natural anti-cancer products: insights from herbal medicine
Chinese Medicine (2025)
-
Lanthanide conjugate Pr-MPO elicits anti-cancer activity by targeting lysosomal machinery and inducing zinc-dependent cataplerosis
Cell Communication and Signaling (2024)
-
The dual role of ferroptosis in anthracycline-based chemotherapy includes reducing resistance and increasing toxicity
Cell Death Discovery (2023)