Abstract
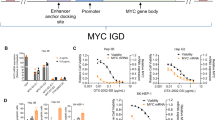
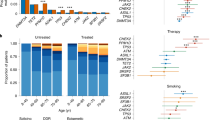
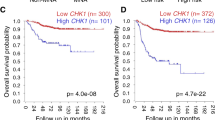
Checkpoint kinase 1 inhibitors (CHK1i) have shown impressive single-agent efficacy in treatment of certain tumors, as monotherapy or potentiators of chemotherapy in clinical trials, but the sensitive tumor types and downstream effectors to dictate the therapeutic responses to CHK1i remains unclear. In this study we first analyzed GDSC (Genomics of Drug Sensitivity in Cancer) and DepMap database and disclosed that hematologic malignancies (HMs) were relatively sensitive to CHK1i or CHK1 knockdown. This notion was confirmed by examining PY34, a new and potent in-house selective CHK1i, which exhibited potent anti-HM effect in vitro and in vivo, as single agent. We demonstrated that the downregulation of c-Myc and its signaling pathway was the common transcriptomic profiling response of sensitive HM cell lines to PY34, whereas overexpressing c-Myc could partially rescue the anticancer effect of PY34. Strikingly, we revealed the significant correlations between downregulation of c-Myc and cell sensitivity to PY34 in 17 HM cell lines and 39 patient-derived cell (PDC) samples. Thus, our results demonstrate that HMs are more sensitive to CHK1i than solid tumors, and c-Myc downregulation could represent the CHK1i efficacy in HMs.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Sanchez Y, Bachant J, Wang H, Hu F, Liu D, Tetzlaff M, et al. Control of the DNA damage checkpoint by chk1 and rad53 protein kinases through distinct mechanisms. Science. 1999;286:1166–71.
Sanchez Y, Wong C, Thoma RS, Richman R, Wu Z, Piwnica-Worms H, et al. Conservation of the Chk1 checkpoint pathway in mammals: linkage of DNA damage to Cdk regulation through Cdc25. Science. 1997;277:1497–501.
Madoz-Gurpide J, Canamero M, Sanchez L, Solano J, Alfonso P, Casal JI. A proteomics analysis of cell signaling alterations in colorectal cancer. Mol Cell Proteom. 2007;6:2150–64.
Sarmento LM, Povoa V, Nascimento R, Real G, Antunes I, Martins LR, et al. CHK1 overexpression in T-cell acute lymphoblastic leukemia is essential for proliferation and survival by preventing excessive replication stress. Oncogene. 2015;34:2978–90.
Boudny M, Zemanova J, Khirsariya P, Borsky M, Verner J, Cerna J, et al. Novel CHK1 inhibitor MU380 exhibits significant single-agent activity in TP53-mutated chronic lymphocytic leukemia cells. Haematologica. 2019;104:2443–55.
Cole KA, Huggins J, Laquaglia M, Hulderman CE, Russell MR, Bosse K, et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc Natl Acad Sci USA. 2011;108:3336–41.
Tho LM, Libertini S, Rampling R, Sansom O, Gillespie DA. Chk1 is essential for chemical carcinogen-induced mouse skin tumorigenesis. Oncogene. 2012;31:1366–75.
Chen Z, Xiao Z, Chen J, Ng SC, Sowin T, Sham H, et al. Human chk1 expression is dispensable for somatic cell death and critical for sustaining G2 DNA damage checkpoint. Mol Cancer Ther. 2003;2:543.
Wang WT, Catto JWF, Meuth M. Differential response of normal and malignant urothelial cells to CHK1 and ATM inhibitors. Oncogene. 2014;34:2887–96.
David L, Fernandez-Vidal A, Bertoli S, Grgurevic S, Lepage B, Deshaies D, et al. CHK1 as a therapeutic target to bypass chemoresistance in AML. Sci Signal. 2016;9:ra90.
Meng Y, Chen CW, Yung MMH, Sun W, Sun J, Li Z, et al. DUOXA1-mediated ROS production promotes cisplatin resistance by activating ATR-Chk1 pathway in ovarian cancer. Cancer Lett. 2018;428:104–16.
Alsubhi N, Middleton F, Abdel-Fatah TM, Stephens P, Doherty R, Arora A, et al. Chk1 phosphorylated at serine345 is a predictor of early local recurrence and radio-resistance in breast cancer. Mol Oncol. 2016;10:213–23.
Fang Z, Gong C, Yu S, Zhou W, Hassan W, Li H, et al. NFYB-induced high expression of E2F1 contributes to oxaliplatin resistance in colorectal cancer via the enhancement of CHK1 signaling. Cancer Lett. 2018;415:58–72.
Mouw KW, Konstantinopoulos PA. From checkpoint to checkpoint: DNA damage ATR/Chk1 checkpoint signalling elicits PD-L1 immune checkpoint activation. Br J Cancer. 2018;118:933–5.
Lee J-M, Nair J, Zimmer A, Lipkowitz S, Annunziata CM, Merino MJ, et al. Prexasertib, a cell cycle checkpoint kinase 1 and 2 inhibitor, in BRCA wild-type recurrent high-grade serous ovarian cancer: a first-in-class proof-of-concept phase 2 study. Lancet Oncol. 2018;19:207–15.
Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Res. 2013;41:D955–61.
Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, et al. Defining a cancer dependency map. Cell. 2017;170:564–76.
Tong L, Song P, Jiang K, Xu L, Jin T, Wang P, et al. Discovery of (R)-5-((5-(1-methyl-1H-pyrazol-4-yl)-4-(methylamino) pyrimidin-2-yl) amino)-3-(piperidin-3-yloxy) picolinonitrile, a novel CHK1 inhibitor for hematologic malignancies. Eur J Med Chem. 2019;173:44–62.
Chang K, Marran K, Valentine A, Hannon GJ. Creating an miR30-based shRNA vector. Cold Spring Harb Protoc. 2013;2013:631–5.
Shen A, Wang L, Huang M, Sun J, Chen Y, Shen YY, et al. c-Myc alterations confer therapeutic response and acquired resistance to c-Met inhibitors in MET-addicted cancers. Cancer Res. 2015;75:4548–59.
Liu H, Ai J, Shen A, Chen Y, Wang X, Peng X, et al. c-Myc alteration determines the therapeutic response to FGFR inhibitors. Clin Cancer Res. 2017;23:974–84.
Chamoun K, Borthakur G. Investigational CHK1 inhibitors in early stage clinical trials for acute myeloid leukemia. Expert Opin Investig Drugs. 2018;27:661–6.
Schuler F, Weiss JG, Lindner SE, Lohmuller M, Herzog S, Spiegl SF, et al. Checkpoint kinase 1 is essential for normal B cell development and lymphomagenesis. Nat Commun. 2017;8:1697.
Bryant C, Scriven K, Massey AJ. Inhibition of the checkpoint kinase Chk1 induces DNA damage and cell death in human leukemia and lymphoma cells. Mol Cancer. 2014;13:147.
Sampath D, Cortes J, Estrov Z, Du M, Shi Z, Andreeff M, et al. Pharmacodynamics of cytarabine alone and in combination with 7-hydroxystaurosporine (UCN-01) in AML blasts in vitro and during a clinical trial. Blood. 2006;107:2517–24.
Sen T, Tong P, Stewart CA, Cristea S, Valliani A, Shames DS, et al. CHK1 inhibition in small-cell lung cancer produces single-agent activity in biomarker-defined disease subsets and combination activity with cisplatin or olaparib. Cancer Res. 2017;77:3870–84.
Hoglund A, Nilsson LM, Muralidharan SV, Hasvold LA, Merta P, Rudelius M, et al. Therapeutic implications for the induced levels of Chk1 in Myc-expressing cancer cells. Clin Cancer Res. 2011;17:7067–79.
Ho JS, Ma W, Mao DY, Benchimol S. p53-dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol. 2005;25:7423–31.
Wang WJ, Wu SP, Liu JB, Shi YS, Huang X, Zhang QB, et al. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013;73:1219–31.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81673466, 81821005, 21772174, and 81172929), the “Personalized Medicines-Molecular Signature-based Drug Discovery and Development” and Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12020214), the National Science and Technology Major Project of China (2018ZX09711002-011-015), and the Science and Technology Commission of Shanghai Municipality (16431902200).
Author information
Authors and Affiliations
Contributions
KLJ, YBZ, TL, JMY, and JL conceived and designed the study. KLJ, YBZ, LX, JL, KXZ, NS, JNL, JYL, MMZ, WBW, and YQX developed the methodology. KLJ, LXT, TW, HLW, XBH, GYX, TTJ, and WJK acquired the data (provided compounds and animals, enrolled and managed patients, provided facilities, etc.). KLJ, HLW, and YBZ analyzed and interpreted the data (e.g., statistical analysis, biostatistics, computational analysis). KLJ and YBZ wrote, reviewed, and/or revised the manuscript. XBH, GYX, TTJ, and WJK supplied administrative, technical, or material support (i.e., reporting or organizing data and constructing databases). AHG, YZH, YBZ, TL, JMY, and JL contributed to the study supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Jiang, Kl., Tong, Lx., Wang, T. et al. Downregulation of c-Myc expression confers sensitivity to CHK1 inhibitors in hematologic malignancies. Acta Pharmacol Sin 43, 220–228 (2022). https://doi.org/10.1038/s41401-021-00652-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00652-1
Keywords
This article is cited by
-
Dual inhibition of CHK1/FLT3 enhances cytotoxicity and overcomes adaptive and acquired resistance in FLT3-ITD acute myeloid leukemia
Leukemia (2023)
-
Functions of lncRNA DUXAP8 in non-small cell lung cancer
Molecular Biology Reports (2022)