Abstract
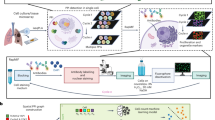
Gene therapy, epigenetic therapies, natural compounds targeted therapy, photodynamic therapy, nanoparticles, and precision medicines are becoming available to diagnose and treat cancer. Gracillin, a natural steroidal saponin extracted from herbs, has shown potent efficacy against a range of malignancies. In this study, we investigated the molecular anticancer mechanisms of gracillin. We showed that gracillin dose-dependently suppressed proliferation, migration, and invasion in breast cancer, liver cancer, and glioblastoma cells with IC50 values around 1 μM, which were associated with MST-independent activation of Hippo signaling pathway and subsequent decreased YAP activity. We demonstrated that gracillin activated the Hippo signaling by inducing Merlin/LATS protein-protein interaction (PPI). A competitive inhibitory peptide (SP) derived from the binding interface of the PPI, disrupted the interaction, abolishing the anticancer activity of gracillin. In nude mice bearing MDA-MB-231, HCCLM3, or U87MG xenograft tumor, administration of gracillin (5, 10 mg·kg−1·d−1, i.g. for 21 days) dose-dependently suppressed the tumor growth, associated with the induced Merlin/LATS PPI, activated Hippo signaling, as well as decreased YAP activity in tumor tissues. Our data demonstrate that gracillin is an efficacious therapeutic agent for cancer treatment, induction of Merlin/LATS PPI might provide proof-of-concept in developing therapeutic agent for cancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Kaur R, Bhardwaj A, Gupta S. Cancer treatment therapies: traditional to modern approaches to combat cancers. Mol Biol Rep. 2023;50:9663–76.
Wang Y, Zhang Q, Chen Y, Liang CL, Liu H, Qiu F, et al. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed Pharmacother. 2020;121:109570.
Charmsaz S, Prencipe M, Kiely M, Pidgeon GP, Collins DM. Innovative technologies changing cancer treatment. Cancers. 2018;10:208.
Charmsaz S, Collins DM, Perry AS, Prencipe M. Novel strategies for cancer treatment: highlights from the 55th IACR Annual Conference. Cancers. 2019;11:1125.
Yang J, Li ZD, Hou CY, Li ZY, Li Q, Miao SY, et al. EM-2 inhibited autophagy and promoted G(2)/M phase arrest and apoptosis by activating the JNK pathway in hepatocellular carcinoma cells. Acta Pharmacol Sin. 2021;42:1139–49.
Zhang H, Xie F, Yuan XY, Dai XT, Tian YF, Sun MM, et al. Discovery of a nitroaromatic nannocystin with potent in vivo anticancer activity against colorectal cancer by targeting AKT1. Acta Pharmacol Sin. 2024;45:1044–59.
Fan S, Qi M, Qi Q, Miao Q, Deng L, Pan J, et al. Targeting FAPα-positive lymph node metastatic tumor cells suppresses colorectal cancer metastasis. Acta Pharm Sin B. 2024;14:682–97.
Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm. 2015;93:52–79.
Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: Treating cancer with specificity. Eur J Pharmacol. 2018;834:188–96.
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6:201.
Huang M, Lin Y, Wang C, Deng L, Chen M, Assaraf YG, et al. New insights into antiangiogenic therapy resistance in cancer: mechanisms and therapeutic aspects. Drug Resist Updates. 2022;64:100849.
Szulzewsky F, Holland EC, Vasioukhin V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev Biol. 2021;475:205–21.
Nguyen CDK, Yi C. YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer. 2019;5:283–96.
Casati G, Giunti L, Iorio AL, Marturano A, Galli L, Sardi I. Hippo pathway in regulating drug resistance of glioblastoma. Int J Mol Sci. 2021;22:13431.
Sebio A, Matsusaka S, Zhang W, Yang D, Ning Y, Stremitzer S, et al. Germline polymorphisms in genes involved in the Hippo pathway as recurrence biomarkers in stages II/III colon cancer. Pharmacogenomics J. 2016;16:312–9.
Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell. 2015;163:811–28.
Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505.
Zhang L, Yang S, Chen X, Stauffer S, Yu F, Lele SM, et al. The hippo pathway effector YAP regulates motility, invasion, and castration-resistant growth of prostate cancer cells. Mol Cell Biol. 2015;35:1350–62.
Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803.
Magesh S, Cai D. Roles of YAP/TAZ in ferroptosis. Trends Cell Biol. 2022;32:729–32.
Guerrero PA, Yin W, Camacho L, Marchetti D. Oncogenic role of Merlin/NF2 in glioblastoma. Oncogene. 2015;34:2621–30.
Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–30.
Mota M, Metge BJ, Hinshaw DC, Alsheikh HA, Chen D, Samant RS, et al. Merlin deficiency alters the redox management program in breast cancer. Mol Oncol. 2021;15:942–56.
Mota MSV, Jackson WP, Bailey SK, Vayalil P, Landar A, Rostas JW, et al. Deficiency of tumor suppressor Merlin facilitates metabolic adaptation by co-operative engagement of SMAD-Hippo signaling in breast cancer. Carcinogenesis. 2018;39:1165–75.
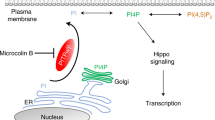
Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–55.
Li Y, Zhou H, Li F, Chan SW, Lin Z, Wei Z, et al. Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell Res. 2015;25:801–17.
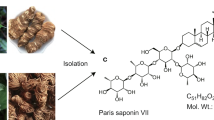
Bhuia MS, Chowdhury R, Sonia FA, Kamli H, Shaikh A, El-Nashar HAS, et al. Anticancer potential of the plant-derived saponin gracillin: a comprehensive review of mechanistic approaches. Chem Biodivers. 2023;20:e202300847.
Marciani DJ. Elucidating the mechanisms of action of saponin-derived adjuvants. Trends Pharmacol Sci. 2018;39:573–85.
Li JK, Zhu PL, Wang Y, Jiang XL, Zhang Z, Zhang Z, et al. Gracillin exerts anti-melanoma effects in vitro and in vivo: role of DNA damage, apoptosis and autophagy. Phytomedicine. 2023;108:154526.
Li Y, Liu H, Liu X, Xiao B, Zhang M, Luo Y, et al. Gracillin shows potential efficacy against non-small cell lung cancer through inhibiting the mTOR pathway. Front Oncol. 2022;12:851300.
Min HY, Jang HJ, Park KH, Hyun SY, Park SJ, Kim JH, et al. The natural compound gracillin exerts potent antitumor activity by targeting mitochondrial complex II. Cell Death Dis. 2019;10:810.
Yang L, Zhu T, Ye H, Shen Y, Li Z, Chen L, et al. Gracillin shows potent efficacy against colorectal cancer through inhibiting the STAT3 pathway. J Cell Mol Med. 2021;25:801–12.
Pearson JD, Huang K, Pacal M, McCurdy SR, Lu S, Aubry A, et al. Binary pan-cancer classes with distinct vulnerabilities defined by pro- or anti-cancer YAP/TEAD activity. Cancer Cell. 2021;39:1115–34.e12.
Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–86.
Rausch V, Hansen CG. The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 2020;30:32–48.
Qian H, Ding CH, Liu F, Chen SJ, Huang CK, Xiao MC, et al. SRY-Box transcription factor 9 triggers YAP nuclear entry via direct interaction in tumors. Signal Transduct Target Ther. 2024;9:96.
Zhang XW, Zhou JC, Peng D, Hua F, Li K, Yu JJ, et al. Disrupting the TRIB3-SQSTM1 interaction reduces liver fibrosis by restoring autophagy and suppressing exosome-mediated HSC activation. Autophagy. 2020;16:782–96.
Parambil ST, Thankayyan SKR, Antony GR, Littleflower AB, Augustine P, Somanathan T, et al. YAP transduction drives triple-negative breast cancer aggressiveness through modulating the EGFR‒AKT axis in patient-derived xenograft cells. Med Oncol. 2023;40:137.
Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell. 2018;173:321–37.e10.
Cha JH, Chan LC, Song MS, Hung MC. New approaches on cancer immunotherapy. Cold Spring Harb Perspect Med. 2020;10:a036863.
Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the treatment of cancer. J Interferon Cytokine Res. 2019;39:6–21.
Li T, Li Y. Quercetin acts as a novel anti-cancer drug to suppress cancer aggressiveness and cisplatin-resistance in nasopharyngeal carcinoma (NPC) through regulating the yes-associated protein/Hippo signaling pathway. Immunobiology. 2023;228:152324.
Najafi S, Majidpoor J, Mortezaee K. Extracellular vesicle-based drug delivery in cancer immunotherapy. Drug Deliv Transl Res. 2023;13:2790–806.
Lv B, Wang Y, Ma D, Cheng W, Liu J, Yong T, et al. Immunotherapy: reshape the tumor immune microenvironment. Front Immunol. 2022;13:844142.
Fan F, He Z, Kong LL, Chen Q, Yuan Q, Zhang S, et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci Transl Med. 2016;8:352ra108.
Rimel BJ, Crane EK, Hou J, Nakayama J, MacDonald J, Lutz K, et al. Tyrosine kinase inhibitor toxicities: A society of gynecologic oncology review and recommendations. Gynecol Oncol. 2023;174:148–56.
Luo HC, Wu JJ, Zhu LJ, Cai LJ, Feng J, Shen ZY, et al. Real-world treatment patterns and survival for locally advanced esophageal squamous cell carcinoma. Medicine. 2023;102:e34647.
Luo M, Xu Y, Chen H, Wu Y, Pang A, Hu J, et al. Advances of targeting the YAP/TAZ-TEAD complex in the hippo pathway for the treatment of cancers. Eur J Med Chem. 2022;244:114847.
Zhou T, Li X, Liu J, Hao J. The Hippo/YAP signaling pathway: the driver of cancer metastasis. Cancer Biol Med. 2023;20:483–9.
Moloudizargari M, Asghari MH, Nabavi SF, Gulei D, Berindan-Neagoe I, Bishayee A, et al. Targeting Hippo signaling pathway by phytochemicals in cancer therapy. Semin Cancer Biol. 2022;80:183–94.
Bachir S, Shah S, Shapiro S, Koehler A, Mahammedi A, Samy RN, et al. Neurofibromatosis Type 2 (NF2) and the implications for vestibular schwannoma and meningioma pathogenesis. Int J Mol Sci. 2021;22:690.
Laraba L, Hillson L, de Guibert JG, Hewitt A, Jaques MR, Tang TT, et al. Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma. Brain. 2023;146:1697–713.
Sekido Y. Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol Int. 2011;61:331–44.
Su T, Ludwig MZ, Xu J, Fehon RG. Kibra and Merlin activate the Hippo pathway spatially distinct from and independent of expanded. Dev Cell. 2017;40:478–90.e3.
Tang R, Chen P, Wang Z, Wang L, Hao H, Hou T, et al. Characterizing the stabilization effects of stabilizers in protein-protein systems with end-point binding free energy calculations. Brief Bioinforma. 2022;23:bbac127.
Guillory X, Wolter M, Leysen S, Neves JF, Kuusk A, Genet S, et al. Fragment-based differential targeting of PPI stabilizer interfaces. J Med Chem. 2020;63:6694–707.
Konstantinidou M, Arkin MR. Molecular glues for protein-protein interactions: Progressing toward a new dream. Cell Chem Biol. 2024;31:1064–88.
Schäfer SC, Voll AM, Bracher A, Ley SV, Hausch F. Antascomicin B stabilizes FKBP51-Akt1 complexes as a molecular glue. Bioorg Med Chem Lett. 2024;104:129728.
Xiang YC, Peng P, Liu XW, Jin X, Shen J, Zhang T, et al. Paris saponin VII, a Hippo pathway activator, induces autophagy and exhibits therapeutic potential against human breast cancer cells. Acta Pharmacol Sin. 2022;43:1568–80.
Peng P, Ren Y, Wan F, Tan M, Wu H, Shen J, et al. Sculponeatin A promotes the ETS1-SYVN1 interaction to induce SLC7A11/xCT-dependent ferroptosis in breast cancer. Phytomedicine. 2023;117:154921.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81973341), the Guangdong Basic and Applied Basic Research Foundation (2024A1515013108), and the Medical Joint Fund of Jinan University (YXJC2022002).
Author information
Authors and Affiliations
Contributions
JXS designed the protocols, performed experiments, drew the figures, and wrote the manuscript. HXZ, ZJZ, and XFZ performed experiments. QMZ, SJL, JQL, SYY, YQL, and RJY contributed to the data collection. XSZ, KC, and HXP provided technical support. ZSL, and HYT contributed to data analysis. MC, YY, QQ, and YBZ funding acquisition, project administration, supervision, revise the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Su, Jx., Zhou, Hx., Zhang, Zj. et al. Gracillin suppresses cancer progression through inducing Merlin/LATS protein-protein interaction and activating Hippo signaling pathway. Acta Pharmacol Sin 46, 2016–2028 (2025). https://doi.org/10.1038/s41401-025-01514-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-025-01514-w