Abstract
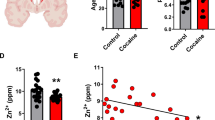
Multiple lines of evidence show that the microRNA system plays a prominent role in regulating behavioral responses to psychostimulants. Suppressing microRNA degradation is an effective strategy for elucidating the impact of these intracellular messengers on cellular function. The translin/trax complex is an RNase that appears to mediate degradation of a small number of microRNAs. In this study we investigated the effect of deleting the translin/trax microRNA-degrading enzyme on cocaine-induced behavioral responses in mice. Wild type and Translin (Tsn) KO mice were injected with cocaine and their open-field locomotor activity was monitored. We found that the locomotor activity in response to repeated (5, 10 and 20 mg/kg, i.p.), but not acute (20 mg/kg, i.p.), cocaine exposure was significantly impaired in Tsn KO mice. We identified several microRNAs (412–5p, 412–3p, 93–3p, 7b–3p, and 204–5p) that were significantly increased in the NAc of Tsn KO mice. As regulator of G-protein signaling 8 (RGS8) is a predicted target gene shared by three of these microRNAs, and expressed in the NAc, we confirmed its reduced expression in this region in Tsn KO mice. Moreover, shRNA-mediated knockdown of RGS8 in the NAc attenuated locomotor sensitization to repeated cocaine administration. Taken together, our results suggest that microRNAs targeted by the translin/trax RNase inhibit cocaine-induced locomotor sensitization, in part, by silencing expression of RGS8.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Schaefer A, Im HI, Veno MT, Fowler CD, Min A, Intrator A, et al. Argonaute 2 in dopamine 2 receptor-expressing neurons regulates cocaine addiction. J Exp Med. 2010;207:1843–51.
Bastle RM, Oliver RJ, Gardiner AS, Pentkowski NS, Bolognani F, Allan AM, et al. In silico identification and validation of miR-495 as a novel regulator of motivation for cocaine that targets multiple addiction-related networks in the nucleus accumbens. Mol Psychiatr. 2018;23:434–43.
Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Guo ML, et al. Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol Neurobiol. 2018;55:3196–210.
Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–7.
Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, et al. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202.
Asada K, Canestrari E, Fu XP, Li Z, Makowski E, Wu YC, et al. Rescuing dicer defects via inhibition of an anti-dicing nuclease. Cell Rep. 2014;9:1471–81.
Fu XP, Shah A, Baraban JM. Rapid reversal of translational silencing: Emerging role of microRNA degradation pathways in neuronal plasticity. Neurobiol Learn Mem. 2016;133:225–32.
Shah AP, Johnson MD, Fu XP, Boersma GJ, Shah M, Wolfgang MJ, et al. Deletion of translin (Tsn) induces robust adiposity and hepatic steatosis without impairing glucose tolerance. Int J Obes. 2020;44:254–66.
Fu XP, Shah AP, Li Z, Li MN, Tamashiro KL, Baraban JM. Genetic inactivation of the translin/trax microRNA-degrading enzyme phenocopies the robust adiposity induced by Translin(Tsn) deletion. Mol Metab. 2020;40:101013.
Tuday E, Nomura Y, Ruhela D, Nakano M, Fu XP, Shah A, et al. Deletion of the microRNA-degrading nuclease, translin/trax, prevents pathogenic vascular stiffness. Am J Physiol-Heart C. 2019;317:H1116–H24.
Tuday E, Nakano M, Akiyoshi K, Fu XP, Shah AP, Yamaguchi A, et al. Degradation of premature-miR-181b by the Translin/Trax RNase increases vascular smooth muscle cell stiffness. Hypertension. 2021;78:831–9.
Wu YC, Williamson R, Li Z, Vicario A, Xu J, Kasai M, et al. Dendritic trafficking of brain-derived neurotrophic factor mRNA: regulation by translin-dependent and -independent mechanisms. J Neurochem. 2011;116:1112–21.
Stein JM, Bergman W, Fang YS, Davison L, Brensinger C, Robinson MB, et al. Behavioral and neurochemical alterations in mice lacking the RNA-binding protein translin. J Neurosci. 2006;26:2184–96.
Okuda A, Kishi T, Okochi T, Ikeda M, Kitajima T, Tsunoka T, et al. Translin-Associated Factor X Gene (TSNAX) may be associated with female major depressive disorder in the Japanese population. Neuromol Med. 2010;12:78–85.
Arias B, Fabbri C, Serretti A, Drago A, Mitjans M, Gastó C, et al. DISC1-TSNAX and DAOA genes in major depression and citalopram efficacy. J Affect Disord. 2014;168:91–7.
Park AJ, Havekes R, Fu XP, Hansen R, Tudor JC, Peixoto L, et al. Learning induces the translin/trax RNase complex to express activin receptors for persistent memory. Elife. 2017;6:e27872.
Park AJ, Shetty MS, Baraban JM, Abel T. Selective role of the translin/trax RNase complex in hippocampal synaptic plasticity. Mol Brain. 2020;13:145.
Fu XP, Shah AAP, Keighron J, Mou TCM, Ladenheim B, Alt J, et al. Elevated body fat increases amphetamine accumulation in brain: evidence from genetic and diet-induced forms of adiposity. Transl Psychiat. 2021;11:427.
Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol. 2003;479:153–8.
Sulzer D, Sonders MS, Poulsen NW, Galli A. Mechanisms of neurotransmitter release by amphetamines: A review. Prog Neurobiol. 2005;75:406–33.
Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–92.
Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98.
Fukuda Y, Ishida R, Aoki K, Nakahara K, Takashi T, Mochida K, et al. Contribution of translin to hematopoietic regeneration after sublethal ionizing irradiation. Biol Pharm Bull. 2008;31:207–11.
Chandra R, Francis TC, Konkalmatt P, Amgalan A, Gancarz AM, Dietz DM, et al. Opposing role for Egr3 in nucleus accumbens cell subtypes in cocaine action. J Neurosci. 2015;35:7927–37.
Carey RJ, DePalma G, Damianopoulos E. Acute and chronic cocaine behavioral effects in novel versus familiar environments: open-field familiarity differentiates cocaine locomotor stimulant effects from cocaine emotional behavioral effects. Behav Brain Res. 2005;158:321–30.
Bailey J, Ma D, Szumlinski KK. Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol. 2012;17:248–58.
Wu J, McCallum SE, Glick SD, Huang Y. Inhibition of the mammalian target of rapamycin pathway by rapamycin blocks cocaine-induced locomotor sensitization. Neuroscience. 2011;172:104–9.
Li H, Zhang J, Xu R, Wang Y, Xu W, Chen R, et al. mTOR regulates cocaine-induced behavioural sensitization through the SynDIG1-GluA2 interaction in the nucleus accumbens. Acta Pharmacol Sin. 2022;43:295–306.
Wu QW, Kapfhammer JP. Modulation of Increased mGluR1 Signaling by RGS8 protects purkinje cells from dendritic reduction and could be a common mechanism in diverse forms of spinocerebellar ataxia. Front Cell Dev Biol. 2021;8:569889.
Loweth JA, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol. 2013;23:500–6.
Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91.
Pierce R, Bell K, Duffy P, Kalivas P. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–60.
Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–65.
Peart DR, Andrade AK, Logan CN, Knackstedt LA, Murray JE. Regulation of cocaine-related behaviours by estrogen and progesterone. Neurosci Biobehav Rev. 2022;135:104584.
Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, et al. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron. 2004;42:269–81.
Williams JM, Steketee JD. Time-dependent effects of repeated cocaine administration on dopamine transmission in the medial prefrontal cortex. Neuropharmacology. 2005;48:51–61.
Argilli E, Sibley DR, Malenka RC, England PM, Bonci A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci. 2008;28:9092–100.
Huang CC, Lin HJ, Hsu KS. Repeated cocaine administration promotes long-term potentiation induction in rat medial prefrontal cortex. Cereb Cortex. 2007;17:1877–88.
Traynor J. Regulator of G protein-signaling proteins and addictive drugs. Ann N Y Acad Sci. 2010;1187:341–52.
Acknowledgements
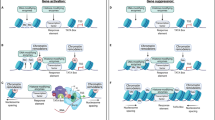
This study was supported by extramural funds from NIDA, the Mid-Atlantic Nutrition Obesity Research Center. Working model created with Generic Diagramming Platform (https://biogdp.com/).
Author information
Authors and Affiliations
Contributions
XPF contributed to the design of experiments, the acquisition, analysis, and interpretation of the data, and the writing of the manuscript. RKW, APS, BL, JA, RC, JLC, RR, XBC contributed to data acquisition and analysis, revision of the manuscript. JMB was responsible for overseeing all parts of the study and directly contributed to the interpretation of the results and writing of the paper. All co-authors provided final approval of the manuscript to be published.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. None of the authors have any other financial disclosures related to the subject matter of this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fu, Xp., Wu, Rk., Shah, A.P. et al. Translin deletion impairs cocaine-induced locomotor sensitization and RGS8 expression in the nucleus accumbens. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01565-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01565-z