Abstract
While observation is the current standard of care for smoldering multiple myeloma (sMM), emerging evidence suggests that early therapeutic intervention may delay progression and improve outcomes especially for high-risk patients. A systematic review and meta-analysis of randomized clinical trials was performed according to the PRISMA guidelines in order to evaluate the effect of treatment compared to observation in outcomes of patients with sMM. Five studies (7 articles) involving 844 patients with intermediate or high risk sMM and comparing treatment to observation or placebo were deemed eligible. All studies reported progression-free survival results, with progression defined as time to active myeloma (without high heterogeneity, I2 = 42%, p = 0.14). A statistically significant 60% reduced pooled risk for disease progression or death (HR = 0.40, 95%CI:0.29–0.55) was revealed for patients who underwent treatment compared to those who did not. An exploratory sensitivity analysis involving 3 trials with only observation in the control group, revealed a 66% lower risk for disease progression or death (HR = 0.34, 95%CI: 0.21–0.56) for patients in the treatment group compared to the control group. Furthermore, time-to-progression was reported in 3 studies; the pooled effect estimate revealed a statistically significant 58% reduced risk for progression to symptomatic MM (HR = 0.42, 95%CI: 0.29–0.61) for patients who underwent treatment compared to those who did not. Only 2 trials reported mature overall survival outcomes, and the pooled effect estimate showed a 45% lower risk for death (HR = 0.55, 95%CI: 0.37–0.82) for sMM patients who received treatment compared to those on observation. Regarding safety, the odds for serious adverse events for those on treatment was as 3.5 times as high (OR = 3.53, 95%CI: 1.14–10.91) compared to those on observation or placebo. In conclusion, this meta-analysis highlights the significant benefits of early treatment in selected patients with sMM, across key clinical outcomes. However, close monitoring is essential for the management of treatment-related toxicities.
Similar content being viewed by others
Background
Smoldering multiple myeloma (sMM) is an intermediate condition between monoclonal gammopathy of undetermined significance (MGUS) and symptomatic multiple myeloma (MM). According to the International Myeloma Working Group (IMWG), it is defined by a clonal plasma cell population in the bone marrow of at least 10% but no more than 60%, and/or a serum monoclonal protein (M-protein) concentration of at least 3 g/dL or urine monoclonal protein (U-protein) concentration of at least 500 mg/24 h, without the co-presence of the conventional CRAB criteria (hypercalcemia, renal failure, anemia, osteolytic bone lesions) or AL amyloidosis or biomarkers indicative of active myeloma (SLiM criteria: 60% or more clonal bone marrow plasma cell percentage, involved to uninvolved free light chain (FLC) ratio 100 or higher, more than 1 focal lesion at least 5 mm on magnetic resonance imaging) [1]. A recent population-level screening study in Iceland indicated that the incidence of sMM among screened individuals aged over 40 years in the general population is 0.53%. Among them, one third were classified as intermediate or high risk for progression to active myeloma [2]. Individuals with sMM are asymptomatic but possess a fluctuating risk of advancing to MM ranging from 10% per year for the first 5 years, to 3% per year for the next 5 years, and 1% per year for the next 10 years [3]. However, high-risk patients present a progression rate of up to 50% within two years [4]. The identification of risk factors, including the percentage of clonal plasma cells in the bone marrow, the level of the M-protein, the involved to uninvolved FLC ratio, the presence of cytogenetic abnormalities such as translocations t(4;14) and t(14;16), 1q addition/amplification, deletion 13q, age, serum creatinine levels and hemoglobin dynamics in time has led to the development of several risk stratification systems that inform physician decisions for patient management [4, 5].
Current guidelines for the management of sMM primarily advocate for observation rather than immediate treatment, given the historically indolent nature of the disease in many patients. The standard of care involves regular monitoring with periodic laboratory studies every 3 to 4 months and yearly imaging assessments with whole-body low-dose computed tomography (WBLDCT) and/or whole body magnetic resonance imaging (WBMRI) to detect progression to symptomatic MM [6]. However, some patients will ultimately behave as having a pre-malignant MGUS and some others as malignant MM [7]. A risk-adapted approach is suggested by consensus recommendations, where low- and intermediate-risk patients undergo observation, while high-risk patients may be considered for participating in clinical trials evaluating early treatment strategies [8]. This conservative strategy is based on the premise that early treatment could expose patients to unnecessary toxicities without proven long-term survival benefits. However, the optimal approach to patients with sMM is evolving as novel prognostic markers and therapeutic interventions emerge.
Recent evidence suggests that early therapeutic intervention with anti-myeloma regimens, particularly for patients with high-risk sMM, may delay disease progression and improve survival outcomes [9,10,11]. These findings challenge the traditional watch-and-wait approach and raise important questions regarding both patient selection and optimal timing of intervention. In this context, this meta-analysis aims to synthesize available data from randomized clinical trials on early therapeutic strategies for patients with sMM, assess their impact on the natural course of the disease and overall survival, and provide a comprehensive evaluation of the potential risks and anticipated benefits.
Methods
The present meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. The study protocol was discussed and agreed upon in advance by all authors.
A systematic search was conducted independently by two authors (INS, CF) in the Medline, ScienceDirect and Cochrane databases from conception until December 16th, 2024 for literature on randomized clinical trials evaluating therapeutic approaches for patients with sMM. The search algorithm encompassed key terms on the disease and on the prespecified study characteristics including the following: (“smoldering multiple myeloma” OR “smouldering multiple myeloma” OR SMM), (“treatment” OR “therapy” OR “management”), (“observation” OR “watch and wait” OR “active surveillance” OR “monitoring” OR “active monitoring” OR “placebo”).
Eligible articles included peer-reviewed full-texts of randomized clinical trials on anti-myeloma therapies for sMM, controlled with observation or no anti-myeloma treatment comparator arms, which reported survival outcomes (PFS, OS) and/or time to progression to symptomatic disease (TTP), in terms of effect outcomes between arms. These were the main outcomes for this study. Secondary outcomes included key safety characteristics, such as grade 3–4 adverse events in total or grade 3–4 hematological adverse events. Case-control, cohort and cross-sectional studies, case series and case reports, reviews, in vitro and animal studies were not included in this meta-analysis.
The present systematic review and meta-analysis was registered at the PROSPERO database with ID CRD42024625308.
Data abstraction and effect estimates
The data abstraction encompassed: general information (first author’s name, publication year, database and clinical trial ID), study characteristics (phase, blinding, follow-up, geographic region, number of participants, number of males, age, risk stratification), intervention characteristics (experimental and control arm treatment), efficacy outcomes (TTP, PFS, OS) and key safety outcomes (rates of serious adverse events (SAEs), neutropenia, thrombocytopenia and infections). Extracted effect estimates included hazard ratios (HR) alongside their 95% confidence intervals (CI), per outcome. If one of the above was not found in the main article, the Supplementary Material was thoroughly screened. There was no shortage of required data for the purposes of the meta-analysis. Data were independently extracted, analyzed and recorded by two authors (INS, CF). The finalized data form was reached after team consensus.
Statistical analyses
Statistical analyses included pooling of studies as well as meta-regressions. Common and Random-effects models were appropriately used to calculate the pooled effect estimates (Proportions, HRs and ORs). Between-study heterogeneity was assessed by Q-test and I2 estimations. When heterogeneity was not low (I2 > 40%), random-effect models results were deemed appropriate. Subgroup and meta-regression analyses were not performed due to the low number of studies included. Throughout the analysis p-values were two sided and the significance level was 0.05. All statistical analyses were performed using R/R-Studio version 2024.04.2 + 764) (Posit Software, PBC).
Assessment of study quality and risk of bias
All records included randomized clinical trials, either blinded or open label. Risk was assessed with the implementation of the RoB:2 by Cochrane to our analysis tools [13]. Publication bias was not possible to be evaluated due to the low number of eligible studies included in the meta-analysis [14,15,16].
Results
Selection of studies
A total of 1568 articles were identified through the search algorithm detailed in the Materials and Methods section. Of these, 299 were deemed as duplicates during the initial screening phase, leaving 1265 records for full-text retrieval. Upon further evaluation, 7 articles were deemed eligible, representing 5 randomized clinical trials investigating different anti-myeloma treatment strategies in patients with sMM compared to observation, placebo or non-anti-myeloma therapy. These trials collectively involved 844 patients [9,10,11, 17,18,19,20]. Fig. 1 illustrates the step-by-step screening process and Table 1 portrays the key study and population characteristics.
Risk stratification of the included patients in eligible studies
Four out of the five clinical trials included exclusively patients with intermediate risk and/or high-risk sMM; however, there were important variations in the definitions among the studies. In the most recent, AQUILA trial [9], high-risk sMM patients were defined those with a percentage of clonal plasma cells in bone marrow of at least 10% and the presence of at least one of the following risk factors: a serum M-protein level of at least 3 g per deciliter, IgA smoldering multiple myeloma, immunoparesis with reduced levels of two uninvolved immunoglobulin isotypes, a ratio of serum involved to uninvolved FLCs (FLC ratio) of 8 to less than 100, or a percentage of clonal plasma cells in bone marrow of more than 50% to less than 60%. In the QuiRedex trial [11, 18, 19], high-risk disease was defined as infiltration of the bone marrow of at least 10% clonal plasma-cells and the presence of a monoclonal component (defined as an IgG level of at least 3 g per deciliter, or an IgA level of at least 2 g per deciliter, or a urinary Bence Jones protein level of more than 1 g per 24 h). If only one of the two abovementioned criteria were present, the patients should have at least 95% phenotypically aberrant plasma cells in the bone marrow plasma cell compartment, along with immunoparesis, defined as the reduction in one or two uninvolved immunoglobulins of more than 25%, as compared with normal values. In the ECOG-ACRIN trial [10], intermediate or high-risk sMM was defined as bone marrow plasmacytosis with 10% or more plasma cells—or sheets of plasma cells—and an abnormal FLC ratio (<0.26 or >1.65) by serum FLC assay. Finally, in the NCT01484275 trial [17], patients with high-risk sMM were defined as having bone marrow clonal plasma cells of at least 10% and either serum M-protein of at least 3 g per deciliter, or abnormal FLC ratio (<0.126 or >8) with serum M-protein more than 1 but less than 3 g per deciliter.
Progression-free survival (PFS)
All of the 5 included studies reported PFS results in terms of treatment effect estimates between arms (HRs). PFS was measured from the date of randomization to the date of the first assessment showing symptomatic disease or death; whichever came first.
Progression to symptomatic MM was defined by the IMWG 2014 SLiM–CRAB criteria [1] in one study [9], and by the 2006 IMWG-CRAB criteria [21] in the other 4 studies [10, 11, 17,18,19,20]. It has to be noted that in the ECOG-ACRIN trial, biochemical progression in combination with CRAB criteria were necessary to document disease progression [22], whereas in the NCT01484275 trial, biochemical progression was considered as a progression event even without the presence of new CRAB features (Table 1).
The pooled overall estimate revealed a statistically significant 60% reduced risk for disease progression or death (HR = 0.40, 95% CI: 0.29–0.55) for patients that underwent anti-myeloma treatment compared to those that did not, while observed heterogeneity was marginally low (I2 = 42%, p = 0.14) (Fig. 2).
In the exploratory sensitivity analysis excluding one trial that was terminated early due to slow accrual [20], all remaining trials compared anti-myeloma treatments with observation (n = 3) or placebo (n = 1) and the pooled risk for disease progression or death was 63% lower (HR = 0.37, 95% CI: 0.25–0.56) for the treatment group compared to the control group (Supplementary Fig. 1). Furthermore, in another exploratory analysis, we also excluded a trial evaluating siltuximab [17], as this drug class has not gained regulatory approval for myeloma treatment. Pooling of the remaining three studies [9,10,11, 18, 19], that evaluated daratumumab monotherapy, lenalidomide monotherapy and the combination of lenalidomide with dexamethasone, showed a 66% lower risk for disease progression or death (HR = 0.34, 95% CI: 0.21–0.56) among patients in the treatment group compared to observation (Supplementary Fig. 2). The PFS benefit from early treatment was even more remarkable when the analysis was confined only to high-risk patients, ie excluding the intermediate risk subgroup from the ECOG study, (HR = 0.28, 95% CI: 0.13–0.62) (Supplementary Fig. 3).
Time to progression (TTP)
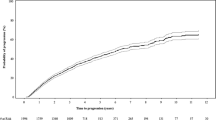
Three out of the 5 included studies reported time-to-progression (TTP) to symptomatic MM in terms of treatment effect estimates between arms (HRs). The pooled overall estimate revealed a statistically significant 58% reduced risk for progression to symptomatic MM (HR = 0.42, 95% CI: 0.29–0.61) for patients that underwent treatment compared to those that did not, while observed heterogeneity was high (I2 = 63%, p = 0.07) (Fig. 3).
Overall response rate (ORR)
Four out of the 5 eligible trials annunciated partial response or better outcomes for the study population under active treatment. The pooled overall response rate (ORR) was 58% (95% CI: 43%–73%) while heterogeneity among the observed proportions was high (I2 = 85%, p < 0.01), as they ranged from 37% to 79% (Fig. 4).
Overall survival
Only 2 trials reported mature OS outcomes as effect estimates between arms (HRs) [9, 11]. Although exploratory, the pooled effect estimate showed a 45% lower risk for death (HR = 0.55, 95% CI: 0.37–0.82) in patients with high-risk sMM who were on treatment compared to those on observation (Supplementary Fig. 3).
Adverse events
All included studies reported grade 3–4 adverse events in each study group. The odds for a grade 3–4 adverse event for those on any anti-myeloma treatment was as 3.5 times as high (OR = 3.53, 95% CI: 1.14, 10.91) compared to those in the control group (Fig. 5A). Moreover, the pooled proportion of grade 3–4 adverse events in the treatment arms was 40% (95% CI: 35%–45%) (Fig. 5B). There were no statistically significant differences observed in the odds of second primary malignancies (SPMs) between treatment and control groups (OR = 1.54, 95% CI: 0.57–4.17) in three studies with pertinent available data [9,10,11] (Fig. 5C).
Quality of life assessment
Two out of the 5 included studies carried out a quality of life assessment and also reported the results in the cited publications. In the AQUILA study [9], patients in both the treatment and observation groups maintained their baseline scores on the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire–Core 30, the EORTC Quality of Life Questionnaire–Multiple Myeloma Module, and the EuroQol 5-Dimension 5-Level questionnaire. Furthermore, the scores obtained during treatment or active monitoring did not differ substantially between the two groups. In the ECOG-ACRIN trial [10], 97% of the patients in the treatment arm and 98% of the patients in the observation arm had baseline health-related quality-of-life data (HRQoL). HRQoL assessment was based on the Functional Assessment of Cancer Therapy-Global (FACT-G: P+F; 14 items, scored 0–56) and the FACT-Multiple Myeloma (FACT-MM;14 items scored 0–56) at study entry, every 6 months and at study discontinuation. No statistically significant differences in the mean change of the scores between the treatment and the control groups were reported at 2 years.
Risk of bias assessment
In the study by Brighton et al. [5], the protocol amendment to reduce the number of patients combined with some key baseline differences, led to an overall high risk of bias in our assessment, as it raised considerable concerns for the randomization process and for deviations from the initially intended interventions. Moreover, the study by Witzig et al. [20] was characterized by a premature interim analysis and trial termination by the independent data monitoring committee due to slow accrual. Furthermore, the inclusion of asymptomatic patients with small lytic lesions that were suspicious for symptomatic MM, was also an important factor that potentially introduced bias. Therefore, this study was also deemed as having an overall high-risk of bias in our assessment. The other three studies demonstrated appropriate protocol adherence and population, intervention and outcomes integrity (Supplementary Table 1). However, in the studies by Lonial et al. [10] and Mateos et al. [11], there were some concerns regarding the robustness of the assessment of the development of symptomatic disease during the study, pertaining to the suboptimal imaging surveillance. In contrast, the study by Dimopoulos et al. [9] had low risk of bias in all evaluated categories as it implemented robust and repeated imaging assessments according to the current standards as proposed by the IMWG. Last but not least, all the studies, including the AQUILA trial, had been designed before the implementation of the Mayo 2018 (20-20-2) risk criteria and the PANGEA models; therefore, they included heterogenous patient populations according to current risk stratification standards.
Discussion
This systematic review and meta-analysis demonstrates that early therapeutic intervention in patients with sMM, who are at elevated risk of progression to symptomatic disease, is beneficial. The pooled analysis revealed a 60% reduction in the risk of disease progression to active myeloma or mortality in patients who received treatment versus those on observation. A more robust effect was observed in studies assessing contemporary anti-myeloma therapeutic regimens like daratumumab and lenalidomide. These findings strengthen the accumulating body of evidence that supports a risk-adapted approach in the management of sMM, thereby transitioning the paradigm from passive surveillance to proactive intervention in those with high-risk characteristics.
Although there is a clear signal for PFS benefit in the majority of the included studies, with progression defined as time to active myeloma, we should note that PFS represents a key surrogate measure for patient-centered outcomes [23, 24]. On the one hand, the therapeutic importance of postponing progression from an asymptomatic state to symptomatic disease may be challenged, especially when considering the lack of evidence for an OS advantage ultimately. Our pooled analysis indicated a 45% reduction in the risk of mortality among treated patients; nevertheless, OS was not a primary study endpoint and the limited number of studies providing mature OS data pinpoints to the need of additional investigations with extended follow-up. On the other hand, our study also indicated that therapy substantially prolonged the TTP to symptomatic multiple myeloma. Administering a fixed-duration and tolerable regimen in order to prevent or postpone end-organ damage is an important element to consider in a shared decision-making process between the clinician and the patient with high-risk sMM. However, we should underline that the progression criteria were not uniformly similar among the included studies in the meta-analysis, which reflects the changing landscape of the management of patients with high-risk sMM over time. All included studies, except for the AQUILA clinical trial [9], had been designed before the implementation of the SLiM-CRAB criteria. Two studies [10, 20] included biochemical criteria similar to symptomatic MM for defining progression in addition to the traditional CRAB features. However, declaring progression in patients with sMM based solely on biochemical progression criteria for MM does not signify true evolution events to symptomatic disease, according to the current knowledge. Defining the optimal endpoint in clinical trials evaluating early intervention in patients with sMM remains challenging. Although the definition of progression to active myeloma according to the SLiM-CRAB criteria may be acceptable from a regulatory perspective, time to event may be substantially prolonged and meaningful results may take years to be reported. In addition, it is important to determine intermediate endpoints, eg biochemical progression or molecular biomarkers, before the documentation of SLiM-CRAB criteria, in order to define a window of opportunity for further early intervention before the occurrence of symptomatic disease.
Another key question in the management of patients with high-risk sMM is whether the aim of treatment should be disease stabilisation or achieving a deep response, with the possibility of eradicating the malignant clone. Although minimal residual disease (MRD) negativity is a well accepted prognostic indicator in patients with symptomatic MM and may drive treatment decisions [25,26,27], its significance in sMM has not been established yet. Single-arm phase 2 studies have shown high rates of sustained MRD negativity with the combination of carfilzomib, lenalidomide and dexamethasone is patients with high-risk sMM [28, 29]. However, the majority of the included randomized studies in the meta-analysis did not consistently evaluate bone marrow MRD or imaging MRD with PET/CT, hence limiting the ability to ascertain whether early intervention may truly prevent in the long-term or only postpones unavoidable development from sMM to MM. This is a crucial issue to address in future research and take into account when designing clinical trials implementing multidrug therapies with curative approach.
Another significant issue in sMM is the variability in risk stratification systems used to identify patients with high-risk disease. While all studies included patients with intermediate- or high-risk sMM, the criteria for risk stratification differed significantly. The AQUILA study [9] included risk variables including levels of M-protein, IgA isotype, immunoparesis, FLC ratio, and bone marrow plasma cells, whereas previous studies such as the QuiRedex [18] and ECOG-ACRIN [10] depended on the levels of involved serum immunoglobulins, proteinuria, aberrant bone marrow plasma cells with immunoparesis, or abnormal FLC ratio. However, none of the included studies implemented the widely accepted 20-20-2 model proposed by the IMWG [4] or the dynamic PANGEA models [5]. Therefore, in the context of the modern risk stratification systems, the population heterogeneity in all the included studies should be acknowledged as a potential source of bias and caution should be made in the interpretation of the results. Furthermore, recent studies have proposed the integration of novel biomarkers, such as circulating tumor cells (CTCs) and immune profiling, in the prognostic models of the evolution of sMM to MM [30,31,32]. Although not universally available, integrating genomic data into the clinical models may enhance the accuracy of risk prediction in SMM. Specific genomic alterations associated with higher risk of progression include mutations in TP53, KRAS, NRAS, MYC, SNORD, BRAF, and the APOBEC system, as well as chromosomal abnormalities such as del(17p), gain(1q), and t(4;14) translocations [33,34,35]. The IMWG has proposed the incorporation of high-risk cytogenetic abnormalities, defined as t(4;14), t(14;16), gain(1q), and/or deletion(13q)/ monosomy 13, in the 20-20-2 model, which discriminates patients with sMM into four risk categories [4]. Gene expression profiling signatures and single-cell RNA sequencing have also demonstrated their ability to characterize high-risk sMM [33, 36,37,38,39]. Overall, this diversity in risk models highlights the need for a standardised definition of high-risk sMM in order to precisely define the patient population that is anticipated to derive benefit from early intervention.
A significant weakness in the included studies was the inconsistent use of imaging techniques both to exclude myeloma bone disease at study entry, and delineate disease progression during treatment and observation. Apart from the AQUILA [9] and the ECOG [9] trials, other studies used traditional skeletal assessments at baseline, a poor method due to its inadequate sensitivity for identifying early signs of myeloma-related bone disease [40, 41]. Although the ECOG study performed a MRI of the spine and pelvis at baseline, this is less sensitive than whole-body MRI (WBMRI) [6, 10]. Intriguingly, one study allowed the inclusion of patients with asymptomatic bone lesions on plain skeletal survey without categorising them as having symptomatic MM [20]. Moreover, the AQUILA study performed comprehensive evaluations for myeloma bone disease with yearly whole body low dose CT (WBLDCT) or CT or PET/CT and MRI of the spine and pelvis every 6 months for the first three years on study and yearly thereafter. In other studies, post baseline imaging was driven by patient symptoms or physician’s discretion. Importantly, up to 10% of patients with sMM may progress solely with bone disease identified by imaging [42, 43]. Prospective evaluation of patients with sMM with modern imaging techniques (WBLDCT, PET/CT, WBMRI) is of outmost importance in order to detect early myeloma-defining bone events and the need for yearly comprehensive assessments is highlighted by the IMWG recommendations [6, 44]. This approach should become the gold standard and be adopted in future trials for patients with sMM.
When discussing potential treatment benefits for patients with otherwise asymptomatic disease, the risk/benefit balance should be considered. As anticipated, our analysis showed an increased frequency of severe toxicities in patients who received active treatment compared to controls. Importantly though, two studies that assessed quality of life indices [9, 10], did not show significant differences between the treatment and observation groups. Furthermore, treatment did not increase the incidence of SPMs. In addition to the above, the decision to provide anti-myeloma treatment in asymptomatic patients with precursor disease entails ethical considerations, in the light of potential overtreatment in individuals who may never develop symptomatic MM. However, high-risk patients have also an increased risk to present with end-organ damage at the time of myeloma progression, even if they are on active surveillance [45,46,47]. Furthermore, the AQUILA study [9] showed that significantly fewer patients required anti-myeloma therapy in the daratumumab group (~20%) than in the observation group (~40%) at 3 years from study entry. Therefore, early intervention prevents/delays a significant number of patients from needing intensive anti-myeloma therapy. For those reasons, it is essential both to evaluate long-term results of ongoing clinical trials and to refine the stratification systems in order to define the true high-risk patients. The incorporation of genomic, proteomic and immunophenotypic features in combination with clinical and laboratory parameters may be useful to this direction [30]. International collaborative efforts are deemed essential to develop and validate new prognostic models for patients with sMM. Overall, our findings underline the need for informed and shared decision making with patients with high risk sMM before initiating treatment in the clinical practice.
In practice, clinical decisions should be tailored, weighting the anticipated benefits of early intervention against the potential hazards of treatment. In our view, a fixed duration therapy with either daratumumab or lenalidomide should be discussed with patients with sMM at high risk for progression to MM according to the currently available stratification systems, in order to reach to a shared decision-making of either early treatment or systematic observation according to the IMWG recommendations.
In conclusion, this meta-analysis collected available evidence from randomized trials on early treatment intervention in patients with high-risk sMM, and showed a PFS benefit with active treatment, with progression defined as time to active myeloma, along with a trend for a potential OS benefit. Nevertheless, taking into consideration the heterogeneity in the design, the inclusion criteria and the outcome assessment among the included studies, the debate is open about the optimal definition of the high-risk sMM, treatment intensity, and long-term patient outcomes.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–548.
Thorsteinsdóttir S, Gíslason GK, Aspelund T, Rögnvaldsson S, Óskarsson JP, Sigurðardóttir GA, et al. Prevalence of smoldering multiple myeloma based on nationwide screening. Nat Med. 2023;29:467–72.
Kyle RA, Remstein ED, Therneau TM, Dispenzieri A, Kurtin PJ, Hodnefield JM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N. Engl J Med. 2007;356:2582–90.
Mateos MV, Kumar S, Dimopoulos MA, Gonzalez-Calle V, Kastritis E, Hajek R, et al. International Myeloma Working Group risk stratification model for smoldering multiple myeloma (SMM). Blood Cancer J. 2020;10:102.
Cowan A, Ferrari F, Freeman SS, Redd R, El-Khoury H, Perry J, et al. Personalised progression prediction in patients with monoclonal gammopathy of undetermined significance or smouldering multiple myeloma (PANGEA): a retrospective, multicohort study. Lancet Haematol. 2023;10:e203–e212.
Hillengass J, Usmani S, Rajkumar SV, Durie BGM, Mateos MV, Lonial S, et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019;20:e302–e312.
Rajkumar SV, Kumar S, Lonial S, Mateos MV. Smoldering multiple myeloma current treatment algorithms. Blood Cancer J. 2022;12:129.
Musto P, Engelhardt M, Caers J, Bolli N, Kaiser M, de Donk NV, et al. 2021 European Myeloma Network review and consensus statement on smoldering multiple myeloma: how to distinguish (and manage) Dr. Jekyll and Mr. Hyde. Haematologica. 2021;106:2799–812.
Dimopoulos MA, Voorhees PM, Schjesvold F, Cohen YC, Hungria V, Sandhu I, et al. Daratumumab or Active Monitoring for High-Risk Smoldering Multiple Myeloma. N Engl J Med. 2025;392:1777–88.
Lonial S, Jacobus S, Fonseca R, Weiss M, Kumar S, Orlowski RZ, et al. Randomized Trial of Lenalidomide Versus Observation in Smoldering Multiple Myeloma. J Clin Oncol. 2020;38:1126–37.
Mateos MV, Hernandez MT, Salvador C, Rubia J, de Arriba F, López-Corral L, et al. Lenalidomide-dexamethasone versus observation in high-risk smoldering myeloma after 12 years of median follow-up time: A randomized, open-label study. Eur J Cancer. 2022;174:243–50.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Murlow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34.
Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. 2018;74:785–94.
Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55.
Brighton TA, Khot A, Harrison SJ, Ghez D, Weiss BM, Kirsch A, et al. Randomized, double-blind, placebo-controlled, multicenter study of siltuximab in high-risk smoldering multiple myeloma. Clin Cancer Res. 2019;25:3772–5.
Mateos MV, Hernandez MT, Giraldo P, de la Rubia J, de Arriba F, Lopez-Corral L, et al. Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N. Engl J Med. 2013;369:438–47.
Mateos MV, Hernandez MT, Giraldo P, de la Rubia J, de Arriba F, orral Lopez-C, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17:1127–36.
Witzig TE, Laumann KM, Lacy MQ, Hayman SR, Dispenzieri A, Kumar S, et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia. 2013;27:220–5.
Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73.
Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P, et al. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22:231–9.
Pawlyn C, Schjesvold FH, Cairns DA, Wei LJ, Davies F, Nadeem O, et al. Progression-free survival as a surrogate endpoint in myeloma clinical trials: an evolving paradigm. Blood Cancer J. 2024;14:134.
Mainou M, Tsapa K, Michailidis T, Malandris K, Karagiannis T, Avgerinos I, et al. Outcomes in randomized controlled trials of therapeutic interventions for multiple myeloma: A systematic review. Crit Rev Oncol Hematol. 2024;204:104529.
Ntanasis-Stathopoulos I, Filippatos C, Ntanasis-Stathopoulos A, Malandrakis P, Kastritis E, Tsitsilonis OE, et al. Evaluating minimal residual disease negativity as a surrogate endpoint for treatment efficacy in multiple myeloma: a meta-analysis of randomized controlled trials. Am J Hematol. 2025;100:427–38.
Landgren O, Prior TJ, Masterson T, Heuck C, Bueno OF, Dash AB, et al. EVIDENCE meta-analysis: evaluating minimal residual disease as an intermediate clinical end point for multiple myeloma. Blood. 2024;144:359–67.
Terpos E, Malandrakis P, Ntanasis-Stathopoulos I, Kostopoulos IV, Eleutherakis-Papaiakovou E, Kanellias N, et al. Sustained bone marrow and imaging MRD negativity for 3 years drives discontinuation of maintenance post ASCT in myeloma. Blood. 2025;145:2353–60.
Mateos MV, Martinez-Lopez J, Rodriguez Otero P, González-Calle V, Gonzalez MS, Oriol A, et al. Curative Strategy for High-Risk Smoldering Myeloma: Carfilzomib, Lenalidomide, and Dexamethasone (KRd) Followed by Transplant, KRd Consolidation, and Rd Maintenance. J Clin Oncol. 2024;42:3247–56.
Kazandjian D, Hill E, Dew A, Morrison C, Roswarski J, Korde N, et al. Carfilzomib, Lenalidomide, and Dexamethasone Followed by Lenalidomide Maintenance for Prevention of Symptomatic Multiple Myeloma in Patients With High-risk Smoldering Myeloma: A Phase 2 Nonrandomized Controlled Trial. JAMA Oncol. 2021;7:1678–85.
Kreiniz N, Gertz MA. Understanding high-risk smoldering multiple myeloma. Leuk Lymphoma. 2023;64:1361–72.
Termini R, Zihala D, Terpos E, Perez-Montaña A, Jelínek T, Raab M, et al. Circulating Tumor and Immune Cells for Minimally Invasive Risk Stratification of Smoldering Multiple Myeloma. Clin Cancer Res. 2022;28:4771–81.
Zavidij O, Haradhvala NJ, Mouhieddine TH, Sklavenitis-Pistofidis R, Cai S, Reidy M, et al. Single-cell RNA sequencing reveals compromised immune microenvironment in precursor stages of multiple myeloma. Nat Cancer. 2020;1:493–506.
Lopez-Corral, Mateos L, Corchete LA MV, Sarasquete ME, de la Rubia J, de Arriba F, et al. Genomic analysis of high-risk smoldering multiple myeloma. Haematologica. 2012;97:1439–43.
Bustoros M, Sklavenitis-Pistofidis R, Park J, Redd R, Zhitomirsky B, Dunford AJ, et al. Genomic Profiling of Smoldering Multiple Myeloma Identifies Patients at a High Risk of Disease Progression. J Clin Oncol. 2020;38:2380–9.
Bolli N, Maura F, Minvielle S, Gloznik D, Szalat R, Fullam A, et al. Genomic patterns of progression in smoldering multiple myeloma. Nat Commun. 2018;9:3363.
Ledergor G, Weiner A, Zada M, Wang SY, Cohen YC, Gatt ME, et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat Med. 2018;24:1867–76.
Boyle EM, Rosenthal A, Ghamlouch H, Wang Y, Farmer P, Rutherford M, et al. Plasma cells expression from smouldering myeloma to myeloma reveals the importance of the PRC2 complex, cell cycle progression, and the divergent evolutionary pathways within the different molecular subgroups. Leukemia. 2022;36:591–5.
Kazandjian D, Diamond B, Papadimitriou M, Hill E, Sklavenitis-Pistofidis R, Ziccheddu B, et al. Genomic Profiling to Contextualize the Results of Intervention for Smoldering Multiple Myeloma. Clin Cancer Res. 2024;30:4482–90.
Botta C, Di Martino MT, Ciliberto D, Cucè M, Correale P, Rossi M, et al. A gene expression inflammatory signature specifically predicts multiple myeloma evolution and patients survival. Blood Cancer J. 2016;6:e511.
Hillengass J, Moulopoulos LA, Delorme S, Koutoulidis V, Mosebach J, Hielscher T, et al. Whole-body computed tomography versus conventional skeletal survey in patients with multiple myeloma: a study of the International Myeloma Working Group. Blood Cancer J. 2017;7:e599.
Gundesen MT, Asmussen JT, Haukas E, Schubert M, Abildgaard N, Schjesvold F, et al. A prospective study of Skeletal survey versus Low-dose whole-body CT for Osteolytic lesions in Multiple Myeloma. Eur J Haematol. 2022;108:423–9.
Gavriatopoulou M, Boultadaki A, Koutoulidis V, Ntanasis-Stathopoulos I, Bourgioti C, Malandrakis P, et al. The Role of Low Dose Whole Body CT in the Detection of Progression of Patients with Smoldering Multiple Myeloma. Blood Cancer J. 2020;10:93.
Ntanasis-Stathopoulos I, Koutoulidis V, Malandrakis P, Fotiou D, Spiliopoulou V, Filippatos C, et al. Yearly Assessment of Bone Disease in Patients with Asymptomatic Multiple Myeloma Identifies Early Progression Events and Should Be the Standard Clinical Practice. J Clin Med. 2025;14:2224.
Kastritis E, Solia I, Malandrakis P, Theodorakakou F, Ntanasis-Stathopoulos I, Kanellias N et al. Patterns of progression among 427 Smoldering Myeloma patients diagnosed after 2014: importance of monitoring. Blood Adv. 2025: bloodadvances.2025016083. Online ahead of print.
Abdallah NH, Lakshman A, Kumar SK, Cook J, Binder M, Kapoor P, et al. Mode of progression in smoldering multiple myeloma: a study of 406 patients. Blood Cancer J. 2024;14:9.
Lakshman A, Rajkumar SV, Buadi FK, Binder M, Gertz MA, Lacy MQ, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018;8:59.
Werly A, Hampel M, Hielscher T, Zuern K, Schmidt SK, Visram A, et al. Patterns of progression in a contemporary cohort of 447 patients with smoldering multiple myeloma. Blood Cancer J. 2024;14:176.
Author information
Authors and Affiliations
Contributions
Conceptualization: INS, MG; Data curation: INS, CF, PM; Formal analysis: CF; Investigation: INS, CF, PM, EK, ET, MAD, MG; Methodology: INS, CF; Supervision: MAD, MG; Roles/Writing - original draft: INS, CF; and Writing - review & editing: PM, EK, ET, MAD, MG.
Corresponding author
Ethics declarations
Competing interests
INS declares honoraria from Janssen. PM declares honoraria from Janssen. EK declares honoraria from Amgen, Janssen, GSK, and Pfizer. ET declares honoraria from Amgen, Astra/Zeneca, Bristol Myers Squibb, Eusa Pharma, GSK, Integris Pharma, Janssen, Pfizer, Sanofi, and Takeda. MAD declares honoraria from Abbvie, Amgen, Bristol Myers Squibb, GSK, Janssen, Karyopharm, Pharmacyclics Inc, Pfizer, Sanofi, and Takeda. MG declares honoraria from GSK, Janssen, Sanofi, Abbvie, Amgen, and Takeda. The other authors declare no conflict of interest.
Ethics approval
An ethics approval was not required for this study as it is a systematic review and meta-analysis that synthesizes previously published, peer-reviewed data.
Informed consent
Informed consent was not required for this study as it is a systematic review and meta-analysis that synthesizes previously published, peer-reviewed data.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ntanasis-Stathopoulos, I., Filippatos, C., Malandrakis, P. et al. Observation or treatment for smoldering multiple myeloma? A systematic review and meta-analysis of randomized controlled studies. Blood Cancer J. 15, 104 (2025). https://doi.org/10.1038/s41408-025-01312-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41408-025-01312-x