Abstract
The treatment of relapsed/refractory acute myeloid leukemia (AML) is associated with a dismal prognosis. The allogeneic hematopoietic cell transplantation (allo-HCT) is frequently performed as salvage therapy. Reduced intensity conditioning protocols have been developed with the aim of reducing the leukemia burden without increasing their toxicity. We compared the reduced intensity conditioning FM140 (fludarabine, 150 mg/m2; melphalan 140 mg/m2) with FBM110 (fludarabine 150 mg/m2; BCNU, also known as carmustine, 300–400 mg/m2; and melphalan 110 mg/m2). From the European Bone Marrow Transplantation (EBMT) Acute Leukemia Working Party registry, we identified 293 adult patients (FM140, n = 118 and FBM110, n = 175) with AML with relapsed/refractory disease prior to allo-HCT. There were some differences such as age (FM140 = 59.5 years vs. FBM110 = 65.1 years, p < 0.001) and graft-versus-host disease (GvHD) prophylaxis based on in vivo T-cell depletion (TCD, FM140 = 39% vs. FBM110 = 75%, p < 0.001). No differences were observed between FM140- and FBM110-treated patients regarding overall survival (OS) (2-year OS: 39.3% vs. 45.7%, p = 0.58), progression-free survival (PFS) (2-year PFS: 36.1% vs. 37.3%, p = 0.69), non-relapse mortality (NRM) (2-year NRM: 15.3% vs. 25.7%, p = 0.10) and relapse incidence (RI) (2-year RI: 48.6% vs. 37.0%, p = 0.7). In conclusion, despite differences in age and GvHD prophylaxis, AML patients with active disease undergoing allo-HCT after FBM110 conditioning showed similar outcomes compared to FM140.
Similar content being viewed by others
Introduction
The treatment of relapsed/refractory AML is associated with a dismal prognosis [1]. The allogeneic hematopoietic cell transplantation (allo-HCT) is frequently performed as salvage therapy in this scenario [2, 3]. Conditioning protocols have been developed with the aim of reducing the leukemia burden without increasing their toxicity, especially in older patients or those with comorbidities [4].
Conditioning with fludarabine/melphalan 140 mg/m2 (FM140) is a standard protocol in many centers for patients with AML in complete remission [5] and in combination with sequential chemotherapy in those with active disease [6, 7]. Compared to a different commonly used protocol based also on an alkylating agent such as fludarabine/busulfan (FluBu2), overall survival (OS) appears to be similar. However, relapse incidence (RI) is lower but non-relapse mortality (NRM) is higher in patients conditioned with FM140 compared to FluBu2 [8,9,10]. Within conditioning protocols with a transplantation conditioning index (TCI) classified as intermediate [11, 12], we have previously shown that patients treated with FM140 have comparable outcomes to those patients treated with the single alkylator-based conditioning protocol, fludarabine/treosulfan (FluTreo) using the registry of the Acute Leukemia Working Party (ALWP) of the European Society for Blood and Marrow Transplantation (EBMT). Patients with FM140 showed a decreased RI and higher NRM resulting in similar OS [13].
In order to increase the anti-leukemic effect without increasing toxicity, conditioning with FM140 has also been modified by adding a second alkylating agent such as BCNU, also known as carmustine, and reducing the melphalan dose (FBM110) [14,15,16]. This conditioning protocol has been shown to have a remarkable anti-leukemic effect including patients with active AML without increasing its toxicity significantly [17, 18]. Similarly, the FTM110 conditioning protocol was established by adding the alkylator thiotepa to the FM140 backbone and reducing the melphalan dose [19]. Patients conditioned with FBM110 and FTM110 showed similar OS after adjusting for clinical variables and these protocols were suitable for older patients and those with comorbidities [20].
In our previous studies, we showed that AML patients in complete remission had better outcomes including OS after conditioning based on two alkylating agents (FBM110/FTM110) compared to single alkylator-based conditioning with FM140 [21]. In this study, using the registry of the EBMT on behalf of the ALWP, we analyzed retrospectively, outcomes of patients with active AML disease, defined as relapsed or refractory, after conditioning with the protocol FM140 compared to FBM110.
Patient and methods
Study design
In this retrospective multicenter analysis, data were provided by the ALWP of the EBMT, who report annually all consecutive allo-HCTs after patient authorization via informed consent. The study was approved by its general assembly. We focused on (1) adult (aged >18 years) patients who received conditioning with FM140 (fludarabine, median 150 mg/m2; melphalan 140 mg/m2) or with FBM110 (fludarabine, median 150 mg/m2; BCNU 300–400 mg/m2 and melphalan 110 mg/m2), (2) first allo-HCT from a matched sibling or unrelated donor for patients with (3) AML in active disease including primary induction failure (PIF), relapsed or progressive disease, (4) transplantation date between January 1st, 2009 and December 31st, 2020 (5) with an unmanipulated peripheral blood graft (no in vitro T-cell depletion (TCD) and no bone marrow grafts). Patients undergoing haploidentical allo-HCT were excluded. We excluded patients, who received high dose melphalan and fludarabine as part from sequential conditioning with f.e. high dose melphalan with FluBu2 or fludarabine/treosulfan (FluTreo) or total body irradiation/fludarabine (TBI/Flu) in order to have a cohort of patients as homogenous as possible and to avoid confounding with different substances and dosages of chemotherapies and TBI. We also excluded patients in CR2/CR3 from the analysis. Hence, we identified 68 patients conditioned with FBM140 and 5 patients with FM110, who were excluded from the analysis. As previously defined [22, 23], patients who never achieved CR despite induction and salvage chemotherapy were classified as having primary refractory AML, while patients who initially achieved CR (BM blasts ≤5%) and then experienced relapse were classified as having relapsing AML. Patients with untreated AML were excluded from the analysis. Cytogenetic risk at diagnosis was categorized according to the 2017 European Leukemia Net (ELN) recommendations for AML [1]. Six centers out of 90 used both protocols. Fifty-nine (20%) of the patients received an unrelated donor graft for which the human leucocyte antigen (HLA) matching was incomplete or had a low resolution making it impossible to calculate the high-resolution mismatches on loci A, B, C, DRB1 and DQB1. These patients were included as unrelated donors. Informed consent was obtained from all patients for use of the clinical data in research.
In contrast to our previous retrospective single-center- and multicenter-based studies [20, 21], patients conditioned with FBM110 and fludarabine, thiotepa, melphalan (FTM110) protocols showed different outcomes and so, we were not able to pool the patients in our single cohort for further analysis.
Statistical analysis
Outcome variables were defined following internal consensus guidelines [24]. Patient-, disease- and treatment-related characteristics were compared using the chi-square test for categorical data or the Mann-Whitney test for continuous data. Baseline characteristics were summarized using median, interquartile range (IQR), and range, for continuous data, and frequency and percentage for categorical data. We assumed a normal distribution of the data and a similar variance in both groups.OS was defined as the time from allo-HCT until death from any cause. Progression-free survival (PFS) was defined as the time from allo-HCT to death from any cause, or relapse/progression, whichever occurred first. Relapse was defined as detection of disease via cytological and/or histological assessment after allo-HCT; death without prior relapse was considered as a competing risk for relapse and was denoted as NRM. For cumulative incidence of acute graft-versus-host disease (aGvHD) and chronic GvHD (cGvHD), death without aGvHD/cGvHD and relapse were considered as competing events. GvHD-free, relapse-free survival (GRFS) was defined as being alive with neither grade III-IV aGVHD nor severe cGVHD, relapse, or death from any cause post-HCT. Patients with no event were censored at the date of last follow-up. To address for the difference in follow-up period between the two conditioning regimen groups, outcome was described at 2 years post transplantation for all comparisons (no censoring at 2 years).
Univariate analyses were performed using Gray’s test for cumulative incidence functions and the log-rank test for OS, GRFS, and LFS. The Cox proportional-hazards model was used for multivariable regression analysis and included variables with unbalanced distribution between the two groups or factors known to predict outcomes. To allow for center differences, a random effect or “frailty” was introduced for each center into the models. Center effect was included in multivariate analysis. Results were expressed as the hazard ratio (HR) with the 95% confidence interval (95% CI).
All tests were two sided. The Type I error was fixed at 0.05 for factors associated with time-to-event outcomes. Statistical analyses were performed with R 4.3.2 (R Development Core Team, Vienna, Austria) software packages.
Results
Patient and transplant characteristics
The patient and transplant characteristics of the 293 AML patients are shown in Table 1. Patients in the FBM110 group were older (65.1 years vs. 59.5 years, p < 0.001), had a lower HCT-CI score (HCT-CI ≥3: 34% vs. 54%, p = 0.02), had more often secondary AML (35% vs. 25%, p = 0.049) and received more often in vivo TCD (92% vs. 78%, p < 0.001). Patients conditioned with FBM110 received more often in vivo TCD based on anti-thymocyte globulin (ATG, 75% vs. 39%) compared to patients conditioned with FM140, who received alemtuzumab more frequently (17% vs. 39%). Other patient and transplant characteristics such as patient sex (p = 0.47), donor sex (p = 0.42), Karnofsky performance status score (KPS) < 90 (p = 0.48), disease status of PIF or relapse (p = 0.95), cytogenetic risk group (p = 0.54), donor type (p = 0.17), neutrophile and thrombocyte engraftment (p = 0.87 and p = 0.08) did not differ between FBM110 and FM140 groups.
Analysis of outcomes in patients conditioned with FM140 compared to FBM110
OS, PFS, RI and NRM
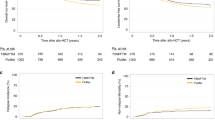
We compared FM140 with FBM110 conditioning in univariate (Table 2, Fig. 1) and multivariate analysis (Table 3). According to multivariate analysis of outcome variables at 2 years, no statistically significant differences were observed between FBM110- compared to FM140-treated patients regarding OS (FBM110 vs. FM140, 45.7% vs. 39.3%, HR in multivariate analysis for FM140 = 0.9, p = 0.58), PFS (37.3% vs 36.1%, HR = 0.93, p = 0.69), NRM 25.7% vs 15.3%, HR = 0.59, p = 0.1) and RI (37% vs. 48.6%, HR = 0.89, p = 0.7).
GvHD and GRFS
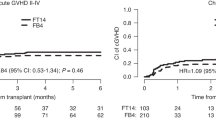
Results regarding GvHD severity and incidence are shown in Tables 2, 3, and Supplementary Table 1. There were no statistically significant differences in 100-day incidence of aGvHD grades II-IV compared to FM140 patients (36.6% vs. 21.6%, HR in multivariate analysis for FM140 = 0.66, p = 0.19), or in 100-day aGvHD grades III-IV (aGvHD III-IV: 15.1% vs. 7.5%, HR = 0.67, p = 0.35) or in 2-year cGvHD (30.8% vs. 28.2%, HR = 0.88, p = 0.53). GFRS at 2 years was also similar between the treatment groups (25.1% vs. 27%, HR 0.8, p = 0.16).
Other variables associated with outcomes
OS, PFS, RI and NRM
We observed that the increment in each year of transplant was positively associated with OS (HR 0.95, p = 0.04) and increasing age by 10 years at transplant was shown to be a favorable factor for OS (HR 0.84, p = 0.046). KPS < 90 was an unfavorable prognostic factor for OS (HR 1.52, p = 0.009) as well as for PFS (HR = 1.44, p = 0.02) and NRM (HR 2.29 p < 0.001). Interestingly, positive CMV status for donor was shown to be a favorable factor for OS and NRM (HR 0.66, p = 0.02; HR 0.49, p = 0.01, respectively), and patient CMV positivity was an unfavorable factor for OS and NRM (HR 1.54, p = 0.02; HR 1.82, p = 0.04, respectively). Use of in vivo TCD as GvHD prophylaxis was shown to be a favorable factor for OS (HR 0.46, p = 0.003) and PFS (HR 0.48 p = 0.004) (Table 3).
GvHD and GRFS
Increasing age by 10 years at transplant was a favorable factor for cGVHD (HR 0.78, p = 0.008) as was in vivo TCD for cGvHD and GFRS (HR 0.53, p = 0.04; HR = 0.4 p = 0.001, respectively). KPS < 90 was associated with increased risk of cGVHD (HR 1.44, p = 0.04) and GRFS (HR 1.53, p = 0.003) (Supplementary Table 1).
Cause of death
Cause of death in patients undergoing allo-HCT conditioned with FM140 compared to FBM110 is described in Supplementary Table 2. Most patients died due to underlying disease (FBM110, n = 58 [53%]; FM140: n = 55 [73%]). Other frequent causes of death were infections (FBM110 n = 19 [17%]; FM140 n = 6 [8%]) and GvHD (FBM110 n = 13 [12%]; FM140 n = 4 [5%]), which were numerically different between the groups.
Discussion
Despite the recent improvements in AML therapies, PIF with relapsed/refractory AML is still clinically challenging and remains an unmet clinical need [25]. Targeted therapies for specific subgroups as well as salvage chemotherapy play a role as bridging therapy to control or reduce AML activity. Nevertheless, an allo-HCT is the only potentially curative option in this scenario.
Modification of conditioning regimens before allo-HCT is a strategy to improve outcome of AML patients with active disease. Sequential chemotherapy with FLAMSA (fludarabine, amsacrine, cytarabine) [26] or TEC (thiotepa, etoposide, cyclophosphamide) [27] as induction chemotherapy followed shortly by a reduced toxicity conditioning has been established as salvage chemotherapy prior to allo-HCT. A randomized clinical trial has recently shown the non-inferiority of undergoing immediately to allo-HCT compared to receiving a high-dose salvage chemotherapy prior to allo-HCT in patients with active AML disease, suggesting patients with relapsed/refractory AML should proceed to allo-HCT as soon as possible [28].
Hence, modifying conditioning chemotherapy prior to allo-HCT has been performed in order to improve outcome of AML patients. Examples of strategies to optimize conditioning therapy include exchanging alkylating chemotherapy with treosulfan [29], adding total body irradiation to sequential chemotherapy conditioning [7, 30] or adding novel therapies such as venetoclax [31] or decitabine [32].
In this retrospective study, we focused on analyzing the outcomes of patients with AML with active disease, including PIF and relapsed/refractory disease, after conditioning with the FBM110 protocol containing two alkylating agents (BCNU and melphalan, FBM110) compared to the backbone protocol with one alkylating agent (melphalan, FM140). Due to the retrospective nature of the study, clinical characteristics were unbalanced between the conditioning groups. Patients conditioned with FBM110 were significantly older, had lower HCT-CI score, suffered more often of secondary AML and received more often in vivo TCD. Hence, the latter patients received more frequently ATG and FM140 patients received alemtuzumab as in vivo TCD.
Although patients conditioned with FBM110 showed numerically less RI at 2 years (48.6% vs. 37%) and increased NRM (25.7% vs. 15.3%), these differences were not statistically significant in multivariate analysis. Therefore, we concluded that despite differences in clinical characteristics such as age and GvHD prophylaxis, patients conditioned with FBM110 or FM140 showed similar outcomes in OS, PFS, RI and NRM. Here, we speculate that the graft vs leukemia effect seems to be independent of a second alkylating agent. Hence, the incidence of aGvHD, cGVHD and GFRS was also similar between both conditioning groups.
Interestingly, patients with a ten year increase in age have significantly better overall survival in multivariate analysis (Table 3, HR 0.84, CI 0.71-1, p = 0.046). Although counterintuitive at first, we speculate that younger patients, not fit for a myeloablative conditioning, receive a conditioning with a TCI intermediate score as FM140 or FBM110. These younger unfit patients have a worse overall survival that older fit patients, who receive standard FM140 or FBM110, suggesting that fitness including co-morbidities and Karnofsky performance score are so, or even more, important than age determining outcome, as recently suggested [33]. We also speculate that the older patients might have received novel therapies as hypomethylating agents in combination with venetoclax, which preserve their fitness prior allo-HCT [34, 35]. In contrast, to younger patients which might have received more toxic induction chemotherapy regimens prior allo-HCT.
Despite not observing statistically significant differences between patients conditioned with FBM110 compared to FM140, it is remarkable that about 45% in the FBM110 group and 40% of the patients in the FM140 group are alive after 2 years and about 30% of the patients in each group are still alive 5 years post allo-HCT. This indicates allo-HCT as an effective therapy in patients with relapsed/refractory AML even at advanced age or with co-morbidities that should be considered in this unfavorable condition.
Donors with CMV status positive were associated with an improved OS and reduced NRM. Furthermore, patients with CMV status positive were associated with a decreased OS and increased NRM. We speculate that donors with CMV donor positive have already immunity against CMV and transplanted patients have less CMV reactivation after alloHCT. Future studies should analyze, if this observation is still currently valid in the era of CMV prophylaxis with letermovir in high risk patients for CMV reactivation after allo-HCT.
This study has some limitations. First, this is a retrospective study of patients included in the EBMT ALWP registry from several centers, mostly in Europe. The gold standard in order to compare two different conditioning regimens is the randomized controlled trial. However, such studies are very expensive to conduct and are very time- and resource-consuming. Second, It is possible, that our statistical analysis did not have enough power to detect subtle differences due to the number of patients (n = 293). Third, the two cohorts had unbalanced patient characteristics e.g. age, and GvHD prophylaxis regimens, which might have influenced outcomes. The EBMT ALWP registry does not have data about % of blasts and mutation profile, which might have differed between the groups. Finally, both protocols are center- and country-specific. FM140 was used more frequently in Great Britain (n = 43, 36%), Germany (n = 28, 24%) and Belgium (n = 21, 18%) and FBM110 was used almost exclusively in Germany (n = 170, 97%). This might account for differences in country-specific transplantation practices not described in the data collected by the EBMT.
Our results show that intensification of chemotherapy-based conditioning regimens with the addition of a second alkylating chemotherapy (BCNU) to melphalan in a dosage of 110 mg/m2 (FBM110) prior to allo-HCT is highly efficient but may not further improve the outcome of patients with AML in active disease compared to melphalan in a dosage of 140 mg/m2 (FM140). To which extent other combination partners (f.e. thiotepa) with melphalan bring an advantage remains speculative. Future strategies for the treatment of patients with relapsed/refractory AML should include novel strategies prior to and after allo-HCT, including targeted therapies, GvHD prophylaxis, novel conditioning protocols and prophylactic infusions of donor lymphocytes. Alternative strategies focused on maintenance therapy after allo-HCT (measurable residual disease-guided) with targeted therapies [36] as well as use of hypomethylating agents with or without venetoclax [37,38,39,40], specific antibodies [41, 42] should also be considered.
In conclusion, despite differences in age, HCT-CI score, frequency in sAML and GvHD prophylaxis based on in vivo TCD, AML patients with relapsed/refractory disease undergoing allo-HCT after FBM110 and FM140 conditioning show similar outcomes. We speculate that the reduced dose of melphalan is compensated by the addition of the second alkylating agent (BCNU) in FBM110 without increasing significantly toxicity or RI. Allo-HCT with toxicity-reduced conditioning is an effective therapy for older patients or those with comorbidities, with relapsed or chemotherapy refractory AML, and it should be considered in this patient population. Future studies should address the efficacy of melphalan-containing regimens in combination with other drugs and/or use of different dosing.
Data availability
The datasets and computer codes generated during and/or analyzed during the current study are available upon reasonable request from the corresponding authors.
References
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Nagler A, Labopin M, Tischer J, Raiola AM, Kunadt D, Vydra J, et al. Haploidentical transplantation in primary refractory/relapsed secondary versus de novo AML: from the ALWP/EBMT. Blood Adv. 2024;8:4223–33.
Mozaffari Jovein M, Ihorst G, Duque-Afonso J, Wäsch R, Bertz H, Wehr C, et al. Long-term follow-up of patients with acute myeloid leukemia undergoing allogeneic hematopoietic stem cell transplantation after primary induction failure. Blood Cancer J. 2023;13:179.
Maffini E, Labopin M, Kröger N, Finke J, Stelljes M, Schroeder T, et al. Allogeneic hematopoietic cell transplantation for older patients with AML with active disease. A study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2024;59:983–90.
van Besien K, Artz A, Smith S, Cao D, Rich S, Godley L, et al. Fludarabine, melphalan, and alemtuzumab conditioning in adults with standard-risk advanced acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol. 2005;23:5728–38.
Ringdén O, Labopin M, Schmid C, Sadeghi B, Polge E, Tischer J, et al. Acute Leukaemia Working Party of the EBMT. Sequential chemotherapy followed by reduced-intensity conditioning and allogeneic haematopoietic stem cell transplantation in adult patients with relapse or refractory acute myeloid leukaemia: a survey from the Acute Leukaemia Working Party of EBMT. Br J Haematol. 2017;176:431–9.
Steckel NK, Groth C, Mikesch JH, Trenschel R, Ottinger H, Kordelas L, et al. High-dose melphalan-based sequential conditioning chemotherapy followed by allogeneic haematopoietic stem cell transplantation in adult patients with relapsed or refractory acute myeloid leukaemia. Br J Haematol. 2018;180:840–53.
Shimoni A, Hardan I, Shem-Tov N, Rand A, Herscovici C, Yerushalmi R, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21:2109–16.
Baron F, Labopin M, Peniket A, Jindra P, Afanasyev B, Sanz MA, et al. Reduced-intensity conditioning with fludarabine and busulfan versus fludarabine and melphalan for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer. 2015;121:1048–55.
Jain T, Alahdab F, Firwana B, Sonbol MB, Almader-Douglas D, Palmer J. Choosing a Reduced-Intensity Conditioning Regimen for Allogeneic Stem Cell Transplantation, Fludarabine/Busulfan versus Fludarabine Melphalan: A Systematic Review and Meta-Analysis. Biol Blood Marrow Transpl. 2019;25:728–33.
Spyridonidis A, Labopin M, Gedde-Dahl T, Ganser A, Stelljes M, Craddock C, et al. Validation of the transplant conditioning intensity (TCI) index for allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2024;59:217–23.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25.
Duque-Afonso J, Finke J, Labopin M, Craddock C, Protheroe R, Kottaridis P, et al. Comparison of fludarabine-melphalan and fludarabine-treosulfan as conditioning prior to allogeneic hematopoietic cell transplantation-a registry study on behalf of the EBMT Acute Leukemia Working Party. Bone Marrow Transpl. 2022;57:1269–76.
Marks R, Potthoff K, Hahn J, Ihorst G, Bertz H, Spyridonidis A, et al. Reduced-toxicity conditioning with fludarabine, BCNU, and melphalan in allogeneic hematopoietic cell transplantation: particular activity against advanced hematologic malignancies. Blood. 2008;112:415–25.
Wais V, Kündgen L, Bohl SR, von Harsdorf S, Schlenk RF, Döhner K, et al. Reduced-toxicity conditioning for allogeneic hematopoietic cell transplantation in elderly or comorbid patients with AML using fludarabine, BCNU and melphalan: disease stage at transplant determines outcome. Bone Marrow Transpl. 2018;53:94–6.
Slack JL, Dueck AC, Fauble VD, Sproat LO, Reeder CB, Noel P, et al. Reduced toxicity conditioning and allogeneic stem cell transplantation in adults using fludarabine, carmustine, melphalan, and antithymocyte globulin: outcomes depend on disease risk index but not age, comorbidity score, donor type, or human leukocyte antigen mismatch. Biol Blood Marrow Transpl. 2013;19:1167–74.
Spyridonidis A, Bertz H, Ihorst G, Grüllich C, Finke J. Hematopoietic cell transplantation from unrelated donors as an effective therapy for older patients (> or = 60 years) with active myeloid malignancies. Blood. 2005;105:4147–8.
Bertz H, Lübbert M, Ohneberg K, Zeiser R, Wäsch R, Marks R, et al. Allogeneic hematopoietic cell transplantation with double alkylating agents containing reduced-intensity conditioning for patients ⩾60 years with advanced AML/MDS. Leukemia. 2016;30:2426–9.
Ciurea SO, Saliba R, Rondon G, Pesoa S, Cano P, Fernandez-Vina M, et al. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transpl. 2010;45:429–36.
Duque-Afonso J, Ihorst G, Waterhouse M, Zeiser R, Wäsch R, Bertz H, et al. Comparison of reduced-toxicity conditioning protocols using fludarabine, melphalan combined with thiotepa or carmustine in allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2021;56:110–20.
Duque-Afonso J, Finke J, Ngoya M, Galimard JE, Craddock C, Raj K, et al. Comparison of fludarabine/melphalan (FluMel) with fludarabine/melphalan/BCNU or thiotepa (FBM/FTM) in patients with AML in first complete remission undergoing allogeneic hematopoietic stem cell transplantation - a registry study on behalf of the EBMT Acute Leukemia Working Party. Bone Marrow Transpl. 2024;59:247–54.
Brissot E, Labopin M, Ehninger G, Stelljes M, Brecht A, Ganser A, et al. Haploidentical versus unrelated allogeneic stem cell transplantation for relapsed/refractory acute myeloid leukemia: a report on 1578 patients from the Acute Leukemia Working Party of the EBMT. Haematologica. 2019;104:524–32.
Poiani M, Labopin M, Battipaglia G, Beelen DW, Tischer J, Finke J, et al. The impact of cytogenetic risk on the outcomes of allogeneic hematopoietic cell transplantation in patients with relapsed/refractory acute myeloid leukemia: On behalf of the acute leukemia working party (ALWP) of the European group for blood and marrow transplantation (EBMT). Am J Hematol. 2021;96:40–50.
Kanate AS, Nagler A, Savani B. Summary of Scientific and Statistical Methods, Study Endpoints and Definitions for Observational and Registry-Based Studies in Hematopoietic Cell Transplantation. Clin Hematol Int. 2019;2:2–4.
Thol F, Döhner H, Ganser A. How I treat refractory and relapsed acute myeloid leukemia. Blood. 2024;143:11–20.
Craddock C, Jackson A, Loke J, Siddique S, Hodgkinson A, Mason J, et al. Augmented Reduced-Intensity Regimen Does Not Improve Postallogeneic Transplant Outcomes in Acute Myeloid Leukemia. J Clin Oncol. 2021;39:768–78.
Duléry R, Ménard AL, Chantepie S, El-Cheikh J, François S, Delage J, et al. Sequential Conditioning with Thiotepa in T Cell- Replete Hematopoietic Stem Cell Transplantation for the Treatment of Refractory Hematologic Malignancies: Comparison with Matched Related, Haplo-Mismatched, and Unrelated Donors. Biol Blood Marrow Transpl. 2018;24:1013–21.
Stelljes M, Middeke JM, Bug G, Wagner-Drouet EM, Müller LP, Schmid C, et al. Remission induction versus immediate allogeneic haematopoietic stem cell transplantation for patients with relapsed or poor responsive acute myeloid leukaemia (ASAP): a randomised, open-label, phase 3, non-inferiority trial. Lancet Haematol. 2024;11:e324–35.
Lazzari L, Ruggeri A, Lupo Stanghellini MT, Mastaglio S, Messina C, Giglio F, et al. Treosulfan-Based Conditioning Regimen Prior to Allogeneic Stem Cell Transplantation: Long-Term Results From a Phase 2 Clinical Trial. Front Oncol. 2021;11:731478.
Stamouli M, Gkirkas K, Karagiannidi A, Iliakis T, Chondropoulos S, Thomopoulos T, et al. Allogeneic Stem Cell Transplantation with a Novel Reduced Intensity Conditioning Regimen for the Treatment of Patients with Primary Cutaneous T-cell Lymphomas. Clin Hematol Int 2021;3:72–6.
Cao XY, Chen JQ, Wang H, Ma W, Liu WW, Zhang FF, et al. Addition of venetoclax to myeloablative conditioning regimens for allogeneic hematopoietic stem cell transplantation in high-risk AML. Ann Med. 2023;55:388–400.
D’Angelo CR, Hall A, Woo KM, Kim K, Longo W, Hematti P, et al. Decitabine induction with myeloablative conditioning and allogeneic hematopoietic stem cell transplantation in high-risk patients with myeloid malignancies is associated with a high rate of infectious complications. Leuk Res. 2020;96:106419.
Saraceni F, Scortechini I, Fiorentini A, Dubbini MV, Mancini G, Federici I, et al. Conditioning Regimens for Frail Patients with Acute Leukemia Undergoing Allogeneic Stem Cell Transplant: How to Strike Gently. Clin Hematol Int. 2021;3:153–60.
Russo D, Polverelli N, Bernardi S, Santarone S, Farina M, Borlenghi E, et al. Venetoclax plus decitabine as a bridge to allogeneic haematopoietic stem-cell transplantation in older patients with acute myeloid leukaemia (VEN-DEC GITMO): final report of a multicentre, single-arm, phase 2 trial. Lancet Haematol. 2024;11:e830–38.
Efficace F, Kicinski M, Coens C, Suciu S, van der Velden WJFM, Noppeney R, et al. Decitabine in older patients with AML: quality of life results of the EORTC-GIMEMA-GMDS-SG randomized phase 3 trial. Blood. 2024;144:541–51.
Xuan L, Wang Y, Yang K, Shao R, Huang F, Fan Z, et al. Sorafenib maintenance after allogeneic haemopoietic stem-cell transplantation in patients with FLT3-ITD acute myeloid leukaemia: long-term follow-up of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. 2023;10:e600–11.
Fathi AT, Kim HT, Soiffer RJ, Levis MJ, Li S, Kim AS, et al. Multicenter Phase I Trial of Ivosidenib as Maintenance Treatment Following Allogeneic Hematopoietic Cell Transplantation for IDH1-Mutated Acute Myeloid Leukemia. Clin Cancer Res. 2023;29:2034–42.
Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv. 2020;4:5580–8.
Gao L, Zhang Y, Wang S, Kong P, Su Y, Hu J, et al. Effect of rhG-CSF Combined With Decitabine Prophylaxis on Relapse of Patients With High-Risk MRD-Negative AML After HSCT: An Open-Label, Multicenter, Randomized Controlled Trial. J Clin Oncol. 2020;38:4249–59.
Kent A, Schwartz M, McMahon C, Amaya M, Smith CA, Tobin J, et al. Venetoclax is safe and tolerable as post-transplant maintenance therapy for AML patients at high risk for relapse. Bone Marrow Transpl. 2023;58:849–54.
Oshikawa G, Kakihana K, Saito M, Aoki J, Najima Y, Kobayashi T, et al. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br J Haematol. 2015;169:756–9.
Apostolova P, Kreutmair S, Toffalori C, Punta M, Unger S, Burk AC, et al. Phase II trial of hypomethylating agent combined with nivolumab for acute myeloid leukaemia relapse after allogeneic haematopoietic cell transplantation-Immune signature correlates with response. Br J Haematol. 2023;203:264–81.
Acknowledgements
The study has been performed on behalf of the ALWP of the EBMT. The authors would like to thank the contribution of the ALWP data managers to this work. According to EBMT rules, co-authorship has been offered to the centers contributing most patients to the analysis. However, we would like to thank all the centers for their contribution. We also thank all the patients and their families.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JD-A, JF, EB, AS, AN, FC and MM designed the study, interpreted the data, and wrote the manuscript. MN and J-EG performed the statistical analysis. JS, MEder, WR, GB, AN, MEdinger, GGW, PJ, HE, MS, DS, EMWD, DB, provided patient data. All the authors critically reviewed the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests with the publication of this article. JD-A has received speaker’s honoraria from Roche, Amgen, AstraZeneca, Beigene, Abbvie, Lilly, Janssen, Riemser, Ipsen, and Sobi and travel support from Alexion, Beigene, Gilead, and Sobi. JF has received research support and speaker’s honoraria from Medac, Neovii, and Riemser. PJ has received speaker’s honoraria from BMS, Abbvie, Janssen, Novartis and travel support from AstraZeneca, Novartis, MSD, Roche. GB has received honoraria from BMS, Gilead, Jazz, Novartis and Otsuka and travel support from Gilead and Jazz. EB received research funding, honorarium, speakers’s fees and travel expenses from Novartis, Astellas, Alexion, Jazz Pharmaceuticals, Gilead, MSD, Keocyt, Amgen, Beigene, Pierre Fabre, Pfizer, Celgene/BMS, Sanofi.
Ethics approval and consent to participate
This study was approved by the general assembly of the ALWP of the EBMT. All methods were performed in accordance with the relevant guidelines and regulations. Patient authorization was performed via informed consent to include their data in the EBMT registry.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duque-Afonso, J., Finke, J., Ngoya, M. et al. Comparison of fludarabine/melphalan (FM140) with fludarabine/melphalan/BCNU (FBM110) in patients with relapsed/refractory AML undergoing allogeneic hematopoietic cell transplantation – a registry study on behalf of the EBMT Acute Leukemia Working Party. Bone Marrow Transplant 60, 373–379 (2025). https://doi.org/10.1038/s41409-024-02499-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02499-6