Abstract
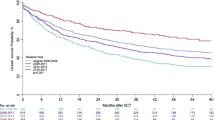
We evaluated brentuximab vedotin (BV) as maintenance therapy after autologous stem cell transplantation (ASCT) in 353 patients with relapsed/refractory Hodgkin lymphoma (HL). Of these, 52.6% received BV prior to ASCT. The five-year overall survival (OS) and progression-free survival (PFS) from the start of BV maintenance were 85.1% and 69.9%, respectively. Multivariable analysis revealed that age at ASCT (HR 1.17, P = 0.037), disease status (HR 3.61, P = 0.002), and BV treatment before ASCT (HR 0.40, P = 0.033) significantly impacted OS. Disease status at ASCT was the only factor significantly associated with PFS (HR 3.09, p < 0.001) and relapse risk (HR 3.33, p < 0.001). Although a trend toward improved PFS (HR 0.59, p = 0.053) and lower relapse risk (HR 0.57, p = 0.051) was observed in patients treated with BV before ASCT, the data were not statistically significant. Patients in complete remission (CR) at ASCT showed similar 2-year OS (94.6% vs. 99.2%, P = 0.3) and PFS (84.6% vs. 89%, P = 0.3) regardless of BV pre-transplant. In those not in CR, OS (83.1% vs. 93.6%, P = 0.076) and PFS (51.5% vs. 75.3%, P = 0.039) were higher in those previously treated with BV. This large study emphasizes BV maintenance post-ASCT, even in patients pre-treated with BV, ang highlights disease status as a key prognostic factor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transpl. 2022;57:1217–39. https://doi.org/10.1038/s41409-022-01691-w.
Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, et al. Long‐term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin’s lymphoma. Biol Blood Marrow Transpl. 2006;12:1065–72.
Hahn T, McCarthy PL, Carreras J, Zhang MJ, Lazarus HM, Laport GG, et al. Simplified validated prognostic model for progression‐free survival after autologous transplantation for Hodgkin lymphoma. Biol Blood Marrow Transpl. 2013;19:1740–44.
Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ, et al. Prognostic factors affecting long‐term outcome after stem cell transplantation in Hodgkin’s lymphoma auto grafted after a first relapse. Ann Oncol. 2005;16:625–633.
Moskowitz CH, Matasar MJ, Zelenetz AD, Nime SD, Gerecitano J, Hamlin P, et al. Normalization of pre‐ASCT, FDG‐PET imaging with second‐line, non‐cross‐resistant, chemotherapy programs improves event‐free survival in patients with Hodgkin lymphoma. Blood. 2012;119:1665–70.
Bröckelmann PJ, Müller H, Casasnovas O, Hutchings M, von Tresckow B, Jürgens M, et al. Risk factors and a prognostic score for survival after autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma. Ann Oncol. 2017;28:1352–58. https://doi.org/10.1093/annonc/mdx072.
Adams HJ, Kwee TC. Prognostic value of pretransplant FDG-PET in refractory/relapsed Hodgkin lymphoma treated with autologous stem cell transplantation: systematic review and meta-analysis. Ann Hematol. 2016;95:695–706. https://doi.org/10.1007/s00277-016-2619-9
Nieto Y, Gruschkus S, Valdez BC, Jones RB, Anderlini P, Hosing C, et al. Improved outcomes of high-risk relapsed Hodgkin lymphoma patients after high-dose chemotherapy: a 15-year analysis. Haematologica. 2022;107:899–908. https://doi.org/10.3324/haematol.2021.278311
Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. AETHERA Study Group. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853–62. https://doi.org/10.1016/S0140-6736(15)60165-9
Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, et al. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132:2639–42. https://doi.org/10.1182/blood-2018-07-861641
Seattle Genetics, Inc. ADCETRISVR (brentuximab vedotin) for Injection. Full prescribing information. Food and Drug Administration. Available at: http://www.seattlegenetics.com/pdf/adcetris_USPI.pdf [Last accessed 20 January 2017].
Takeda Pharma A/S ADCETRISVR 50 mg powder for concentrate for solution for infusion. Summary of Product Characteristics. European Medicines Agency. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human/002455/WC500135055.pdf [Last accessed 20 January 2017].
Massaro F, Pavone V, Stefani PM, Botto B, Pulsoni A, Patti C, et al. Brentuximab vedotin consolidation after autologous stem cell transplantation for Hodgkin lymphoma: A Fondazione Italiana Linfomi real-life experience. Hematol Oncol. 2022;40:31–39. https://doi.org/10.1002/hon.2939
Marouf A, Cottereau AS, Kanoun S, Deschamps P, Meignan M, Franchi P, et al. Outcomes of refractory or relapsed Hodgkin lymphoma patients with post-autologous stem cell transplantation brentuximab vedotin maintenance: a French multicenter observational cohort study. Haematologica. 2022;107:1681–86. https://doi.org/10.3324/haematol.2021.279564
Akay OM, Ozbalak M, Pehlivan M, Yildiz B, Uzay A, Yigenoglu TN, et al. Brentuximab vedotin consolidation therapy after autologous stem-cell transplantation in patients with high-risk Hodgkin lymphoma: Multicenter retrospective study. Hematol Oncol. 2021;39:498–505. https://doi.org/10.1002/hon.2897
Martínez C, de Haro ME, Romero S, Gutiérrez A, Domingo-Domènech E, González-Rodríguez AP, et al. Grupo Español de Linfoma y Trasplante de Médula Ósea (GELTAMO) y Grupo Español de Trasplante (GETH). Impact of pre- and/or post-autologous stem cell transplantation exposure to brentuximab vedotin on survival outcomes in patients with high-risk Hodgkin lymphoma. Ann Hematol. 2023;102:429–37. https://doi.org/10.1007/s00277-022-05011-6.
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–86.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68.
LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, et al. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132:40–48. https://doi.org/10.1182/blood-2017-11-815183
O’Connor OA, Lue JK, Sawas A, Amengual JE, Deng C, Kalac M, et al. Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin’s lymphoma: an international, multicentre, single-arm, phase 1-2 trial. Lancet Oncol. 2018;19:257–66. https://doi.org/10.1016/S1470-2045(17)30912-9
Lynch RC, Cassaday RD, Smith SD, Fromm JR, Cowan AJ, Warren EH, et al. Dose-dense brentuximab vedotin plus ifosfamide, carboplatin, and etoposide for second-line treatment of relapsed or refractory classical Hodgkin lymphoma: a single centre, phase 1/2 study. Lancet Haematol. 2021;8:e562–e571. https://doi.org/10.1016/S2352-3026(21)00170-8
Kersten MJ, Driessen J, Zijlstra JM, Plattel WJ, Morschhauser F, Lugtenburg PJ, et al. Combining brentuximab vedotin with dexamethasone, high-dose cytarabine and cisplatin as salvage treatment in relapsed or refractory Hodgkin lymphoma: the phase II HOVON/LLPC Transplant BRaVE study. Haematologica. 2021;106:1129–37. https://doi.org/10.3324/haematol.2019.243238
Garcia-Sanz R, Sureda A, de la Cruz F, Canales M, Gonzalez AP, Pinana JL, et al. Brentuximab vedotin and ESHAP is highly effective as second-line therapy for Hodgkin lymphoma patients (long-term results of a trial by the Spanish GELTAMO Group). Ann Oncol. 2019;30:612–20. https://doi.org/10.1093/annonc/mdz009
Sureda A, Nunez Céspedes J, Terol MJ, hernández Mohedo F, Domingo-Domènech E, de la Cruz Vicente F, et al. Brentuximab vedotin - ESHAP significantly increases the metabolic complete remission rate versus ESHAP in relapsed classical Hodgkin’s lymphoma. Final results of the BRESELIBET prospective trial. HemaSphere. 2024;8:1983–84.
Moskowitz AJ, Shah G, Schöder H, Ganesan N, Drill E, Hancock H, et al. Phase II Trial of Pembrolizumab Plus Gemcitabine, Vinorelbine, and Liposomal Doxorubicin as Second-Line Therapy for Relapsed or Refractory Classical Hodgkin Lymphoma. J Clin Oncol. 2021;39:3109–17. https://doi.org/10.1200/JCO.21.01056
Bryan LJ, Casulo C, Allen PB, Smith SE, Savas H, Dillehay GL, et al. Pembrolizumab Added to Ifosfamide, Carboplatin, and Etoposide Chemotherapy for Relapsed or Refractory Classic Hodgkin Lymphoma: A Multi-institutional Phase 2 Investigator-Initiated Nonrandomized Clinical Trial. JAMA Oncol. 2023;9:683–91. https://doi.org/10.1001/jamaoncol.2022.7975
Mei MG, Lee HJ, Palmer JM, Chen R, Tsai NC, Chen L, et al. Response-adapted anti-PD-1-based salvage therapy for Hodgkin lymphoma with nivolumab alone or in combination with ICE. Blood. 2022;139:3605–16. https://doi.org/10.1182/blood.2022015423
Armand P, Chen YB, Redd RA, Joyce RM, Bsat J, Jeter E, et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood. 2019;134:22–29. https://doi.org/10.1182/blood.2019000215
Herrera AF, Chen L, Nieto Y, Holmberg L, Johnston P, Mei M, et al. Brentuximab vedotin plus nivolumab after autologous haematopoietic stem-cell transplantation for adult patients with high-risk classic Hodgkin lymphoma: a multicentre, phase 2 trial. Lancet Haematol. 2023;10:e14–e23. https://doi.org/10.1016/S2352-3026(22)00318-0
Falade AS, Redd RA, Shah H, Baron K, Iyengar S, Desai SH, et al. Efficacy of brentuximab vedotin maintenance therapy following autologous stem cell transplantation in patients with relapsed/refractory classical Hodgkin lymphoma with and without pre-transplant exposure to novel agents. Blood. 2023;142:3062–64.
Kanate AS, Kumar A, Dreger P, Dreyling M, Le Gouill S, Corradini P, et al. Maintenance Therapies for Hodgkin and Non-Hodgkin Lymphomas After Autologous Transplantation: A Consensus Project of ASBMT, CIBMTR, and the Lymphoma Working Party of EBMT. JAMA Oncol. 2019;5:715–22. https://doi.org/10.1001/jamaoncol.2018.6278
Author information
Authors and Affiliations
Contributions
CM and AS conceived and designed the study; CM interpreted the statistical results and wrote the manuscript; IK collected and assembled data; MF performed the statistical analysis and interpreted the results; and CM, IK, MF, BDF, AM, HG, LMF, FM, PMS, FM, BB, BF, OMA, MÖ, MEH, SR, JEG, BG, AB, AS are physicians from LWP-EBMT centers who performed the transplants, took care of the patients, collected local data of patients, and made significant contributions to the discussion of the results. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
C. Martínez declares participation in Scientific Takeda Advisory Board. I. Khvedelidze declares no conflict of interest. M. Fekom declares no conflict of interest. B. Deau Fischer declares participation in Scientific Takeda and Abbvie Advisory board. A. Marouf declares no conflict of interest. H. Ghesquières declares Roche, BMS, Takeda consultancy; Honoraria: Gilead, Roche, BMS, Abbvie, Takeda. L.M. Fornecker declares no conflict of interest. F. Merli declares Honoraria/Advisory Board: Beigene, Gilead, Incyte, Janssen, Novartis, Roche, Sandoz, Takeda, Astrazeneca. P. M. Stefani declares participation in Advisory Board: Takeda, Roche, Janssen-Cilag, Incyte. Kiowa Kirin Gilead. F. Massaro declares no conflict of interest. B. Botto declares Takeda Speakers Bureau participation. B. Ferhanoğlu declares no conflict of interest. O. Meltem Akay declares no conflict of interest. M. Özbalak declares no conflict of interest. M. Espeso de Haro declares Research Funding: Pfizer; Advisory Board: Roche, Janssen, Abbvie, Kite, Takeda, Celgene. S. Romero declares no conflict of interest. J.E. Galimard declares no conflict of interest. B. Glass declares Roche, Consultancy, Membership on an entity’s Board ofDirectors or advisory committees, and Research Funding; Gilead, Consultancy and Membership on an entity’s Board ofDirectors or advisory committees; BMS, Consultancy and Membership on an entity’s Board ofDirectors or advisory committees; Miltenyi, Consultancy; Abbvie, Consultancy; Sobie, Consultancy; JAZZ, Honoraria. A. Bazarbachi declares Research support: Takeda, Novartis, Jansen, Roche and Pfizer; Honoraria: Takeda, Amgen, Caribou, Jansen, Roche. A. Sureda declares Takeda Honoraria, Membership on an entity’s Board of Directors or advisory committees and SpeakersBureau; Roche, Honoraria; Novartis, Honoraria and Membership on an entity’s Board ofDirectors or advisory committees; Janssen, Honoraria and Membership on an entity’s Board ofDirectors or advisory committees; BMS, Honoraria and Membership on an entity’s Board ofDirectors or advisory committees.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Martínez, C., Khvedelidze, I., Fekom, M. et al. Outcomes of patients with Hodgkin lymphoma receiving Brentuximab Vedotin (BV) as maintenance therapy after ASCT according to previous exposure to BV. A retrospective analysis of the EBMT Lymphoma Working Party in collaboration with GELTAMO, FIL, LYSA, and Turkish Lymphoma Group. Bone Marrow Transplant 60, 879–887 (2025). https://doi.org/10.1038/s41409-025-02568-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-025-02568-4