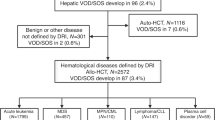
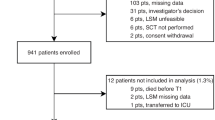
Abstract
Sinusoidal obstruction syndrome (SOS) formerly known as Veno-occlusive disease (VOD) is a potentially fatal complication that occurs mainly after haematopoietic cell transplantation, especially allogeneic transplantation. The liver is the principal organ affected, though other organs, such as the lungs, may also be involved to a lesser extent. The condition is characterised by obstruction of the hepatic venules, leading to sinusoidal congestion, hepatic ischaemia and, in severe cases, fulminant liver failure. Recent refined diagnostic criteria, published by the European Society for Blood and Marrow Transplantation in 2023, provide a more accurate method of detecting SOS/VOD, allowing earlier intervention and better stratification of patients according to the severity of their disease. This article focuses on liver SOS/VOD and discussing key risk factors, new diagnostic methods and therapeutic strategies, with an emphasis on the early use of defibrotide, which remains the reference treatment for severe SOS/VOD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
No clinical data was generated or analyzed in this study.
References
Mohty M, Malard F, Alaskar AS, Aljurf M, Arat M, Bader P, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a refined classification from the European society for blood and marrow transplantation (EBMT). Bone Marrow Transpl. 2023;58:749–54.
Bras G, Hill KR. Veno-occlusive disease of the liver; essential pathology. Lancet. 1956;271:161–3.
Jacobs P, Miller JL, Uys CJ, Dietrich BE. Fatal veno-occlusive disease of the liver after chemotherapy, whole-body irradiation and bone marrow transplantation for refractory acute leukaemia. S Afr Med J. 1979;55:5–10.
Berk PD, Popper H, Krueger GR, Decter J, Herzig G, Graw RG. Veno-occlusive disease of the liver after allogeneic bone marrow transplantation: possible association with graft-versus-host disease. Ann Intern Med. 1979;90:158–64.
McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology. 1984;4:116–22.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–83.
Mohty M, Malard F, Abecassis M, Aerts E, Alaskar AS, Aljurf M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transpl. 2016;51:906–12.
Ichikawa H, Yakushijin K, Kurata K, Tsuji T, Takemoto N, Joyce M, et al. Utility of the refined EBMT diagnostic and severity criteria 2023 for sinusoidal obstruction syndrome/veno-occlusive disease. Bone Marrow Transpl. 2024;59:518–25.
Colecchia A, Ravaioli F, Sessa M, Alemanni VL, Dajti E, Marasco G, et al. Liver stiffness measurement allows early diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome in adult patients who undergo hematopoietic stem cell transplantation: results from a monocentric prospective study. Biol Blood Marrow Transpl. 2019;25:995–1003.
Colecchia A, Marasco G, Ravaioli F, Kleinschmidt K, Masetti R, Prete A, et al. Usefulness of liver stiffness measurement in predicting hepatic veno-occlusive disease development in patients who undergo HSCT. Bone Marrow Transpl. 2017;52:494–7.
Debureaux PE, Bourrier P, Rautou PE, Zagdanski AM, De Boutiny M, Pagliuca S, et al. Elastography improves accuracy of early hepato-biliary complications diagnosis after allogeneic stem cell transplantation. Haematologica. 2021;106:2374–83.
Cañas T, Suárez O, Rozas I, Escribano M, Molina B, González-Vicent M, et al. Point shear-wave elastography for the diagnosis of veno-occlusive disease in children and young adults. Pediatr Radio. 2023;53:2013–20.
Reddivalla N, Robinson AL, Reid KJ, Radhi MA, Dalal J, Opfer EK, et al. Using liver elastography to diagnose sinusoidal obstruction syndrome in pediatric patients undergoing hematopoetic stem cell transplant. Bone Marrow Transpl. 2020;55:523–30.
Inoue Y, Saitoh S, Denpo H, Yamaguchi K, Kubota K, Taya Y, et al. Utility of liver stiffness measurement in the diagnosis of sinusoidal obstruction syndrome/veno-occlusive disease after hematopoietic stem cell transplantation. J Med Ultrason (2001). 2024;51:311–21.
Schulz M, Vuong LG, Müller HP, Maibier M, Tacke F, Blau IW, et al. Shear wave elastography in the detection of sinusoidal obstruction syndrome in adult patients undergoing allogenic hematopoietic stem cell transplantation. Diagnostics (Basel). 2021;11:928.
Lee YS, Lee S, Choi YH, Cho YJ, Lee SB, Cheon JE, et al. Usefulness of two-dimensional shear wave elastography in diagnosing hepatic veno-occlusive disease in pediatric patients undergoing hematopoietic stem cell transplantation. Ultrasonography. 2023;42:286–96.
Vythoulkas D, Tsirigotis P, Griniezaki M, Konstantellos I, Lazana I. Endothelial dysfunction syndromes after allogeneic stem cell transplantation. Cancers (Basel). 2023;15:680.
Cairo MS, Cooke KR, Lazarus HM, Chao N. Modified diagnostic criteria, grading classification and newly elucidated pathophysiology of hepatic SOS/VOD after haematopoietic cell transplantation. Br J Haematol. 2020;190:822–36.
Corbacioglu S, Jabbour EJ, Mohty M. Risk factors for development of and progression of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transpl. 2019;25:1271–80.
Larue M, Labopin M, Brissot E, Alaskar AS, Aljurf M, Arat M, et al. An international survey to better understand the current incidence, severity, and management of VOD/SOS. Bone Marrow Transplant. 2025;60:28–31.
Strouse C, Zhang Y, Zhang MJ, DiGilio A, Pasquini M, Horowitz MM, et al. Risk score for the development of veno-occlusive disease after allogeneic hematopoietic cell transplant. Biol Blood Marrow Transpl. 2018;24:2072–80.
Wallhult E, Kenyon M, Liptrott S, Mank A, Ní Chonghaile M, Babic A, et al. Management of veno-occlusive disease: the multidisciplinary approach to care. Eur J Haematol. 2017;98:322–9.
Hardouin G, Miccio A, Brusson M. Gene therapy for β-thalassemia: current and future options. Trends Mol Med. 2025;31:344–58.
Ballantine J, Tisdale JF. Gene therapy for sickle cell disease: recent advances, clinical trials and future directions. Cytotherapy. 2024:S1465-3249(24)00925-3.
Dimitrievska M, Bansal D, Vitale M, Strouboulis J, Miccio A, Nicolaides KH, et al. Revolutionising healing: gene Editing’s breakthrough against sickle cell disease. Blood Rev. 2024;65:101185.
Frangoul H, Locatelli F, Sharma A, Bhatia M, Mapara M, Molinari L, et al. Exagamglogene autotemcel for severe sickle cell disease. N Engl J Med. 2024;390:1649–62.
Roeker LE, Kim HT, Glotzbecker B, Nageshwar P, Nikiforow S, Koreth J, et al. Early clinical predictors of hepatic veno-occlusive disease/sinusoidal obstruction syndrome after myeloablative stem cell transplantation. Biol Blood Marrow Transpl. 2019;25:137–44.
Putta S, Young B, Pine P, Shi J, Amber V, Saber W, et al. Verification of the prediction accuracy of a biomarker-based prognostic for veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after hematopoietic cell transplantation (HCT). Transpl Cell Ther. 2024;30:986.e1–986.e7.
Jiang S, Penack O, Terzer T, Schult D, Majer-Lauterbach J, Radujkovic A, et al. Predicting sinusoidal obstruction syndrome after allogeneic stem cell transplantation with the EASIX biomarker panel. Haematologica. 2021;106:446–53.
Chan SS, Colecchia A, Duarte RF, Bonifazi F, Ravaioli F, Bourhis JH. Imaging in hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Biol Blood Marrow Transpl. 2020;26:1770–9.
Simpson S, Breshears E, Basavalingu D, Khatri G, Chan S, Fite J, et al. Review of imaging findings in hepatic veno-occlusive disease. Eur J Radio. 2024;177:111526.
Dietrich CF, Bamber J, Berzigotti A, Bota S, Cantisani V, Castera L, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (short version). Ultraschall Med. 2017;38:377–94.
de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–74.
Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127:1656–65.
Richardson PG, Carreras E, Iacobelli M, Nejadnik B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018;2:1495–509.
Nauffal M, Kim HT, Richardson PG, Soiffer RJ, Antin JH, Cutler C, et al. Defibrotide: real-world management of veno-occlusive disease/sinusoidal obstructive syndrome after stem cell transplant. Blood Adv. 2022;6:181–8.
Mohty M, Battista ML, Blaise D, Calore E, Cesaro S, Maximova N, et al. A multicentre, multinational, prospective, observational registry study of defibrotide in patients diagnosed with veno-occlusive disease/sinusoidal obstruction syndrome after haematopoietic cell transplantation: an EBMT study. Bone Marrow Transpl. 2021;56:2454–63.
Corbacioglu S, Topaloglu O, Aggarwal S. A systematic review and meta-analysis of studies of defibrotide prophylaxis for veno-occlusive disease/sinusoidal obstruction syndrome. Clin Drug Investig. 2022;42:465–76.
Mohty M, Blaise D, Peffault de Latour R, Labopin M, Bourhis JH, Bruno B, et al. Real-world use of defibrotide for veno-occlusive disease/sinusoidal obstruction syndrome: the DEFIFrance Registry Study. Bone Marrow Transpl. 2023;58:367–76.
Gómez-Centurión I, Bailén R, Oarbeascoa G, Muñoz C, Luque AÁ, Boyra ME, et al. Transjugular intrahepatic portosystemic shunt for very severe veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after unmanipulated haploidentical hematopoietic stem cell transplantation with post-transplantation cyclophosphamide. Biol Blood Marrow Transpl. 2020;26:2089–97.
Marcoux C, Saliba RM, Wallis W, Khazal S, Ragoonanan D, Rondon G, et al. Incidence and risk factors of early onset VOD/SOS differ in younger vs older adults after stem cell transplantation. Blood Adv. 2024;8:1128–36.
Petrie JR, Guzik TJ, Touyz RM. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol. 2018;34:575–84.
Hebbel RP, Vercellotti GM. Multiple inducers of endothelial NOS (eNOS) dysfunction in sickle cell disease. Am J Hematol. 2021;96:1505–17.
Caruso C, Cheng X, Michaud ME, Szafraniec HM, Thomas BE, Fay ME, et al. Less-deformable erythrocyte subpopulations biomechanically induce endothelial inflammation in sickle cell disease. Blood. 2024;144:2050–62.
Farina M, Scaini MC, Facchinetti A, Leoni A, Bernardi S, Catoni C, et al. Evaluation of circulating endothelial cells as direct marker of endothelial damage in allo-transplant recipients at high risk of hepatic veno-occlusive disease/sinusoidal obstruction syndrome. Transpl Cell Ther. 2024;30:580.e1–580.e14.
Author information
Authors and Affiliations
Contributions
MM and ML conceived and designed the study; MM and ML wrote the manuscript. All authors critically revised and approved the final manuscript. MM. is the guarantor of the study.
Corresponding author
Ethics declarations
Competing interests
AP: Received speaker’s bureau fees from Jazz Pharmaceuticals. FB: Attended AB and received speaker’s fees from Jazz Pharmaceuticals. AA: Received honoraria and consultancy fees from Novartis, AbbVie, Janssen, Takeda, Kyowa Kirin, Gilead, Roche, and Sanofi. Participated in company-sponsored speaker’s bureau for Janssen, Kyowa Kirin, and Sanofi. MK: Received honoraria and consultancy fees from Jazz Pharmaceuticals, Sanofi, Roche, Mallinckrodt, and Vertex. Participated in speaker’s bureau for Jazz Pharmaceuticals, Sanofi, and Pfizer.
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations. - This study did not report any patient data. - Ethical approval was not required for this study as it consists only of expert recommendations and does not involve clinical data, human participants or animal subjects.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Larue, M., Malard, F., Alaskar, A.S. et al. Management of liver sinusoidal obstruction syndrome/veno-occlusive disease in adults: a 2025 perspective from an international expert group. Bone Marrow Transplant (2025). https://doi.org/10.1038/s41409-025-02598-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41409-025-02598-y