Abstract
Background
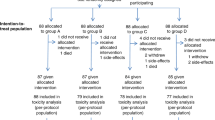
We report the long-term results as primary endpoint in a multicentre randomized prospective Phase 2 trial which compared chemoradiotherapy (CRT) and triplet chemotherapy (CT) as the initial therapy for conversion surgery (CS) in T4b esophageal cancer (EC).
Methods
Patients with T4b EC were randomly assigned to the CRT group or CT group as initial treatment. CS was performed if resectable after initial or secondary treatment. The primary endpoint was 2-year overall survival, analysed by intention-to-treat.
Results
The median follow-up period was 43.8 months. The 2-year survival rate was higher in the CRT group (55.1%; 95% CI: 41.1–68.3%) compared to the CT group (34.7%; 95% CI: 22.8–48.9%), although the difference was not significant (P = 0.11). Local and regional lymph node recurrence in patients undergoing R0 resection was significantly higher in the CT group compared to the CRT group (local: 30% versus 8%, respectively, P = 0.03; regional: 37% versus 8%, respectively, P = 0.002).
Conclusions
Upfront CT was not superior to upfront CRT as induction therapy for T4b EC in terms of 2-year survival and was significantly inferior to upfront CRT in terms of local and regional control.
Registration
The Japan Registry of Clinical Trials (s051180164).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
269,00 € per year
only 11,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gamliel Z, Krasna MJ. Multimodality treatment of esophageal cancer. Surg Clin North Am. 2005;85:621–30.
Tachimori Y, Ozawa S, Numasaki H, Ishirhara R, Matsubara H, Muro K, et al. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus. 2019;16:221–45.
Watanabe M, Tachimori Y, Oyama T, Toh Y, Matsubara H, Ueno M, et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus. 2021;18:1–24.
Watanabe M, Toh Y, Ishihara R, Kono K, Matsubara H, Murakami K, et al. Comprehensive registry of esophageal cancer in Japan, 2014. Esophagus. 2022;19:1–26.
Versteijne E, van Laarhoven HWM, van Hooft JE, van Os RM, Geijsen ED, van Berge Henegouwen MI, et al. Definitive chemoradiation for patients with inoperable and/or unresectable esophageal cancer: locoregional recurrence pattern. Dis Esophagus. 2015;28:453–9.
Gwynne S, Hurt C, Evans M, Holden C, Vout L, Crosby T. Definitive chemoradiation for oesophageal cancer; a standard of care in patients with non-metastatic oesophageal cancer. Clin Oncol. 2011;23:182–188.
Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–21.
Jingu K, Umezawa R, Matsushita H, Sugawara T, Kubozono M, Yamamoto T, et al. Chemoradiotherapy for T4 and/or M1 lymph node esophageal cancer: experience since 2000 at a high-volume center in Japan. Int J Clin Oncol. 2016;21:276–82.
Fujita H, Sueyoshi S, Tanaka T, Tanaka Y, Matono S, Moti N, et al. Esophagectomy: is it necessary after chemoradiotherapy for a locally advanced T4 esophageal cancer? Prospective nonrandomized trial comparing chemoradiotherapy with surgery versus without surgery. World J Surg. 2005;29:25–31.
Shinoda M, Ando N, Kato K, Ishikura S, Kato H, Tsubosa T, et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci. 2015;106:407–12.
Akutsu Y, Kono T, Uesato M, Hoshino I, Kurakami K, Aoyagi T, et al. Is the outcome of a salvage surgery for T4 thoracic esophageal squamous cell carcinoma really poor? World J Surg. 2014;38:2891–7.
de Manzoni G, Pedrazzani C, Pasini F, Bernini M, Maria Minicozzi A, Giacopuzzi S, et al. Chemoradiotherapy followed by surgery for squamous cell carcinoma of the thoracic esophagus with clinical evidence of adjacent organ invasion. J Surg Oncol. 2007;95:261–6.
Pimiento JM, Weber J, Hoffe SE, Shridhar R, Almhanna K, Vignesh S, et al. Outcomes associated with surgery for T4 esophageal cancer. Ann Surg Oncol. 2013;20:2706–12.
Ohkura Y, Ueno M, Iizuka T, Udagawa H. Prognostic factors and appropriate lymph node dissection in salvage esophagectomy for locally advanced T4 esophageal cancer. Ann Surg Oncol. 2019;26:209–16.
Sugawara K, Yagi K, Okumura Y, Nishida M, Aikou S, Yamashita H, et al. Long-term outcomes of multimodal therapy combining definitive chemoradiotherapy and salvage surgery for T4 esophageal squamous cell carcinoma. Int J Clin Oncol. 2020;25:552–60.
Yamasaki M, Miyata H, Tanaka K, Shiraishi O, Motoori M, Peng YF, et al. Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology. 2011;80:307–13.
Yamasaki M, Yasuda T, Yano M, Hirao M, Kobayashi K, Fujitani K, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28:116–20.
Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable esophageal cancer. Br J Cancer. 2016;115:1328–34.
Yokota T, Kato K, Hamamoto Y, Tsubosa Y, Ogawa H, Ito Y, et al. A 3-year overall survival update from a phase 2 study of chemoselection with DCF and subsequent conversion surgery for locally advanced unresectable esophageal cancer. Ann Surg Oncol. 2020;27:460–7.
Satake H, Tahara M, Mochizuki S, Kato K, Hara H, Yokota T, et al. A prospective, multicenter phase I/II study of induction chemotherapy with docetaxel, cisplatin and fluorouracil (DCF) followed by chemoradiotherapy in patients with unresectable locally advanced esophageal carcinoma. Cancer Chemother Pharmacol. 2016;78:91–9.
Sugimura K, Miyata H, Tanaka K, Makino T, Takeno A, Shiraishi O, et al. Randomized controlled trial multicenter randomized phase 2 trial comparing chemoradiotherapy and docetaxel plus 5-fluorouracil and cisplatin (DCF) chemotherapy as initial induction therapy for subsequent conversion surgery in patients with clinical T4b esophageal cancer: short-term results. Ann Surg. 2021;274:e465–72.
Hong SJ, Kim TJ, Nam KB, Lee IS, Yang HC, Cho S, et al. New TNM staging system for esophageal cancer: what chest radiologists need to know. Radiographics. 2014;34:1722–40.
Picus D, Balfe DM, Loehler RE, Roper CL, Owen JW. Computed tomography in the staging of esophageal carcinoma. Radiology. 1983;146:433–8.
Makino T, Yamasaki M, Miyazaki Y, Wada N, Takahashi T, Kurokawa Y, et al. Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (DCF) for T4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2017;31:1–7.
Hashimoto T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, et al. The pattern of residual tumor after neoadjuvant chemotherapy for locally advanced esophageal cancer and its clinical significance. Ann Surg. 2020;271:875–84.
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704.
Stahl M, Stuschke M, Lehmann N, Meyer HJ, Walz MK, Seeber S, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol. 2005;23:2310–7.
Bedenne L, Michel P, Bouche O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol. 2007;25:1160–8.
Miyata H, Yamasaki M, Kurokawa Y, Takiguchi S, Nakajima K, Fujiwara Y, et al. Clinical relevance of induction triplet chemotherapy for esophageal cancer invading adjacent organs. J Surg Oncol. 2012;106:441–7.
Terada M, Hara H, Daiko H, Mizusawa J, Kadota T, Hori K, et al. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol. 2019;49:1055–60.
Noordman BJ, Verdam MGE, Lagarde SM, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, et al. Effect of neoadjuvant chemoradiotherapy on health-related quality of life in esophageal or junctional cancer: results from the randomized CROSS trial. J Clin Oncol. 2018;36:268–75.
Noordman BJ, Verdam MGE, Lagarde SM, Shapiro J, Hulshof MCCM, van Berge Henegouwen MI, et al. Impact of neoadjuvant chemoradiotherapy on health-related quality of life in long-term survivors of esophageal or junctional cancer: results from the randomized CROSS trial. Ann Oncol. 2018;29:445–51.
Kunisaki C, Makino H, Takagawa R, Yamamoto N, Nagano Y, Fujii S, et al. Surgical outcomes in esophageal cancer patients with tumor recurrence after curative esophagectomy. J Gastrointest Surg. 2008;12:802–10.
Sugiyama M, Morita M, Yoshida R, Ando K, Egashira A, Ohga T, et al. Patterns and time of recurrence after complete resection of esophageal cancer. Surg Today. 2012;42:752–8.
Acknowledgements
The authors thank the staff from all centers that participated in data collection.
Funding
This work was supported by a grant-in-aid from Japanese Foundation for Multidisciplinary Treatment of Cancer (KS), a grant-in-aid from The Osaka Community Foundation (KS), a grant-in-aid from The Public Trust Fund for Clinical Cancer Research (KS) and a grant-in-aid from Japan Research Foundation for Clinical Pharmacology (KS).
Author information
Authors and Affiliations
Contributions
Study concepts: M Yano, YD and TY. Development of the methodology, data analysis and interpretation: M Yamasaki, KS, HM, KT, TM, AT, OS, M Motoori, YK, MH, KF, TY, HE, YD and M Yano. Manuscript preparation, editing and review: M Yamasaki. All authors had access to the study data and have reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The human ethics review committee of each institution approved the study protocol. Subjects provided written informed consent. This study was performed in accordance with the Declarations of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yamasaki, M., Miyata, H., Yamashita, K. et al. Chemoradiotherapy versus triplet chemotherapy as initial therapy for T4b esophageal cancer: survival results from a multicenter randomized Phase 2 trial. Br J Cancer 129, 54–60 (2023). https://doi.org/10.1038/s41416-023-02286-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02286-y
This article is cited by
-
Conversion surgery for esophageal and esophagogastric junction cancer
International Journal of Clinical Oncology (2024)