Abstract
Background
Tertiary lymphoid structures (TLSs) impact cancer outcomes, including in triple-negative breast cancer (TNBC), where their role in immune modulation during neoadjuvant therapy (NAT) is underexplored.
Methods
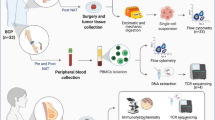
This study employed single-cell RNA sequencing (scRNA-seq), multiplex immunofluorescence (mIF) staining, and radiomic techniques to evaluate TLSs and the tumour microenvironment (TME) in TNBC patient samples before and after NAT.
Results
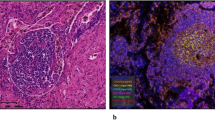
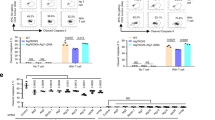
The presence of TLSs in TNBC was associated with B-cell maturation and T-cell activation. Compared with TLS-low TNBC, TLS-high TNBC showed significantly greater expression of immunoglobulin family genes (IGHM and IGHG1) in B cells and greater cytotoxicity of neoantigen-specific CD8 + T cells (neoTCR8). Additionally, mIF revealed notable differences between TLSs and the TME in TNBC. Although CD8 + T-cell levels do not predict the NAT response effectively, TLS maturity strongly correlated with better NAT outcomes and prognosis (P < 0.05). An imaging biomarker scoring system was also developed to predict TLS status and NAT efficacy.
Conclusion
Our results demonstrated changes in TLSs and the TME in TNBC patients post-NAT. These findings confirm the predictive value of mature TLSs (mTLSs) and support the use of personalised immunotherapy based on post-NAT immune characteristics, thereby improving clinical outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
269,00 € per year
only 11,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data are available upon reasonable request. All data relevant to this study are included in the article or uploaded as online supplemental information.
Code availability
The underlying code for this study, along with the associated training and validation datasets, is not publicly available. However, it can be made available to qualified researchers upon a reasonable request to the corresponding author.
References
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15–23.
Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. BMJ. 2023;381:e071674.
Sharma P, Stecklein SR, Yoder R, Staley JM, Schwensen K, O’Dea A, et al. Clinical and Biomarker Findings of Neoadjuvant Pembrolizumab and Carboplatin Plus Docetaxel in Triple-Negative Breast Cancer: NeoPACT Phase 2 Clinical Trial. JAMA Oncol. 2024;10:227–35.
Li Y, Zhang H, Merkher Y, Chen L, Liu N, Leonov S, et al. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol. 2022;15:121.
Kuroda H, Jamiyan T, Yamaguchi R, Kakumoto A, Abe A, Harada O, et al. Tumor-infiltrating B cells and T cells correlate with postoperative prognosis in triple-negative carcinoma of the breast. BMC Cancer. 2021;21:286.
Zhang N-N, Qu F-J, Liu H, Li Z-J, Zhang Y-C, Han X, et al. Prognostic impact of tertiary lymphoid structures in breast cancer prognosis: a systematic review and meta-analysis. Cancer Cell Int. 2021;21:536.
Zhang Q, Wu S. Tertiary lymphoid structures are critical for cancer prognosis and therapeutic response. Front Immunol. 2022;13:1063711.
Wang Q, Sun K, Liu R, Song Y, Lv Y, Bi P, et al. Single-cell transcriptome sequencing of B-cell heterogeneity and tertiary lymphoid structure predicts breast cancer prognosis and neoadjuvant therapy efficacy. Clin Transl Med. 2023;13:e1346.
Pandey PR, Young KH, Kumar D, Jain N. RNA-mediated immunotherapy regulating tumor immune microenvironment: next wave of cancer therapeutics. Mol Cancer. 2022;21:58.
Sun X, Liu W, Sun L, Mo H, Feng Y, Wu X, et al. Maturation and abundance of tertiary lymphoid structures are associated with the efficacy of neoadjuvant chemoimmunotherapy in resectable non-small cell lung cancer. J Immunother Cancer. 2022;10:11.
Siliņa K, Soltermann A, Attar FM, Casanova R, Uckeley ZM, Thut H, et al. Germinal Centers Determine the Prognostic Relevance of Tertiary Lymphoid Structures and Are Impaired by Corticosteroids in Lung Squamous Cell Carcinoma. Cancer Res. 2018;78:1308–20.
Al-Rawi DH, Lettera E, Li J, DiBona M, Bakhoum SF. Targeting chromosomal instability in patients with cancer. Nat Rev Clin Oncol. 2024;21:645–59.
Drews RM, Hernando B, Tarabichi M, Haase K, Lesluyes T, Smith PS, et al. A pan-cancer compendium of chromosomal instability. Nature. 2022;606:976–83.
Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–9.
Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78.
Chen L, Chen L, Ni H, Shen L, Wei J, Xia Y, et al. Prediction of CD3 T cells and CD8 T cells expression levels in non-small cell lung cancer based on radiomic features of CT images. Front Oncol. 2023;13:1104316.
Pan D, Chen H, Xu J, Lin X, Li L. Evaluation of vital genes correlated with CD8 + T cell infiltration as prognostic biomarkers in stomach adenocarcinoma. BMC Gastroenterol. 2023;23:399.
Kang W, Qiu X, Luo Y, Luo J, Liu Y, Xi J, et al. Application of radiomics-based multiomics combinations in the tumor microenvironment and cancer prognosis. J Transl Med. 2023;21:598.
Zhou H, Luo Q, Wu W, Li N, Yang C, Zou L. Radiomics-guided checkpoint inhibitor immunotherapy for precision medicine in cancer: A review for clinicians. Front Immunol. 2023;14:1088874.
Shiao SL, Gouin KH, Ing N, Ho A, Basho R, Shah A, et al. Single-cell and spatial profiling identify three response trajectories to pembrolizumab and radiation therapy in triple negative breast cancer. Cancer Cell. 2024;42:70–84.
Pal B, Chen Y, Vaillant F, Capaldo BD, Joyce R, Song X, et al. A single-cell RNA expression atlas of normal, preneoplastic and tumorigenic states in the human breast. EMBO J. 2021;40:e107333.
Wang B, Liu J, Han Y, Deng Y, Li J, Jiang Y. The Presence of Tertiary Lymphoid Structures Provides New Insight Into the Clinicopathological Features and Prognosis of Patients With Breast Cancer. Front Immunol. 2022;13:868155.
Li R, Berglund A, Zemp L, Dhillon J, Putney R, Kim Y, et al. The 12-CK Score: Global Measurement of Tertiary Lymphoid Structures. Front Immunol. 2021;12:694079.
Zeng Z, Li J, Zhang J, Li Y, Liu X, Chen J, et al. Immune and stromal scoring system associated with tumor microenvironment and prognosis: a gene-based multi-cancer analysis. J Transl Med. 2021;19:330.
Hafemeister C, Satija R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019;20:296.
Isaeva OI, Sharonov GV, Serebrovskaya EO, Turchaninova MA, Zaretsky AR, Shugay M, et al. Intratumoral immunoglobulin isotypes predict survival in lung adenocarcinoma subtypes. J Immunother Cancer. 2019;7:279.
Sun Y-P, Ke Y-L, Li X. Prognostic value of CD8+ tumor-infiltrating T cells in patients with breast cancer: A systematic review and meta-analysis. Oncol Lett. 2023;25:39.
Zheng C, Fass JN, Shih Y-P, Gunderson AJ, Sanjuan Silva N, Huang H, et al. Transcriptomic profiles of neoantigen-reactive T cells in human gastrointestinal cancers. Cancer Cell. 2022;40:410–23.
Bassez A, Vos H, Van Dyck L, Floris G, Arijs I, Desmedt C, et al. A single-cell map of intratumoral changes during anti-PD1 treatment of patients with breast cancer. Nat Med. 2021;27:820–32.
Simoni Y, Becht E, Fehlings M, Loh CY, Koo S-L, Teng KWW, et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature. 2018;557:575–9.
Leem G, Jeon M, Kim KW, Jeong S, Choi SJ, Lee YJ, et al. Tumour-infiltrating bystander CD8+ T cells activated by IL-15 contribute to tumour control in non-small cell lung cancer. Thorax. 2022;77:769–80.
Narvaez D, Nadal J, Nervo A, Costanzo MV, Paletta C, Petracci FE, et al. The Emerging Role of Tertiary Lymphoid Structures in Breast Cancer: A Narrative Review. Cancers (Basel). 2024;16:396.
Kang W, Feng Z, Luo J, He Z, Liu J, Wu J, et al. Tertiary Lymphoid Structures in Cancer: The Double-Edged Sword Role in Antitumor Immunity and Potential Therapeutic Induction Strategies. Front Immunol. 2021;12:689270.
Pusztai L, Yau C, Wolf DM, Han HS, Du L, Wallace AM, et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: Results from the adaptively randomized I-SPY2 trial. Cancer Cell. 2021;39:989–98.
McArthur H, Cortés J, Dent R, O’Shaughnessy J, Pusztai L, Küemmel S, et al. Abstract PD3-01: Neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy followed by adjuvant pembrolizumab vs placebo for early TNBC: Post hoc analysis of adjuvant radiation therapy in the phase 3 KEYNOTE-522 study. Cancer Res. 2023;83(5_Supplement):PD3-01-PD3-01.
Voorwerk L, Slagter M, Horlings HM, Sikorska K, van de Vijver KK, de Maaker M, et al. Immune induction strategies in metastatic triple-negative breast cancer to enhance the sensitivity to PD-1 blockade: the TONIC trial. Nat Med. 2019;25:920–8.
Mariniello A, Nasti TH, Chang DY, Hashimoto M, Malik S, McManus DT, et al. Platinum-Based Chemotherapy Attenuates the Effector Response of CD8 T Cells to Concomitant PD-1 Blockade. Clin Cancer Res. 2024;30:1833–45.
Lu J-C, Wu L-L, Sun Y-N, Huang X-Y, Gao C, Guo X-J, et al. Macro CD5L+ deteriorates CD8+T cells exhaustion and impairs combination of Gemcitabine-Oxaliplatin-Lenvatinib-anti-PD1 therapy in intrahepatic cholangiocarcinoma. Nat Commun. 2024;15:621.
Lu H, Lou H, Wengert G, Paudel R, Patel N, Desai S, et al. Tumor and local lymphoid tissue interaction determines prognosis in high-grade serous ovarian cancer. Cell Rep Med. 2023;4:101092.
Sarradin V, Lusque A, Filleron T, Dalenc F, Franchet C. Immune microenvironment changes induced by neoadjuvant chemotherapy in triple-negative breast cancers: the MIMOSA-1 study. Breast Cancer Res. 2021;23:61.
Zhang H, Ye L, Yu X, Jin K, Wu W. Neoadjuvant therapy alters the immune microenvironment in pancreatic cancer. Front Immunol. 2022;13:956984.
Barmpoutis P, Di Capite M, Kayhanian H, Waddingham W, Alexander DC, Jansen M, et al. Tertiary lymphoid structures (TLS) identification and density assessment on H&E-stained digital slides of lung cancer. PLoS One. 2021;16:e0256907.
Tang R, Xu J, Wang W, Meng Q, Shao C, Zhang Y, et al. Targeting neoadjuvant chemotherapy-induced metabolic reprogramming in pancreatic cancer promotes anti-tumor immunity and chemo-response. Cell Rep Med. 2023;4:101234.
Chen Y, Wu Y, Yan G, Zhang G. Tertiary lymphoid structures in cancer: maturation and induction. Front Immunol. 2024;15:1369626.
Lauss M, Donia M, Svane IM, Jönsson G. B Cells and Tertiary Lymphoid Structures: Friends or Foes in Cancer Immunotherapy? Clin Cancer Res. 2022;28:1751–8.
Liang Y, Lü W, Zhang X, Lü B. Tumor-infiltrating CD8+ and FOXP3+ lymphocytes before and after neoadjuvant chemotherapy in cervical cancer. Diagn Pathol. 2018;13:93.
Dias Costa A, Väyrynen SA, Chawla A, Zhang J, Väyrynen JP, Lau MC, et al. Neoadjuvant Chemotherapy Is Associated with Altered Immune Cell Infiltration and an Anti-Tumorigenic Microenvironment in Resected Pancreatic Cancer. Clin Cancer Res. 2022;28:5167–79.
Lu Y, Zhao Q, Liao J-Y, Song E, Xia Q, Pan J, et al. Complement Signals Determine Opposite Effects of B Cells in Chemotherapy-Induced Immunity. Cell. 2020;180:1081–97.e24.
Panthi B, Adrada BE, Candelaria RP, Guirguis MS, Yam C, Boge M, et al. Assessment of Response to Neoadjuvant Systemic Treatment in Triple-Negative Breast Cancer Using Functional Tumor Volumes from Longitudinal Dynamic Contrast-Enhanced MRI. Cancers (Basel). 2023;15:1025.
Yu Y, Tan Y, Xie C, Hu Q, Ouyang J, Chen Y, et al. Development and Validation of a Preoperative Magnetic Resonance Imaging Radiomics-Based Signature to Predict Axillary Lymph Node Metastasis and Disease-Free Survival in Patients With Early-Stage Breast Cancer. JAMA Netw Open. 2020;3:e2028086.
Acknowledgements
We would like to thank the staff members of the TCGA and GEO Research Network, as well as all of the authors, for making their valuable research data public.
Funding
The present study was supported by the High-level Talent Introduction Project of Fujian Cancer Hospital (No.: F2328R-GC301-01) and the High-level Talent Training Program of Fujian Cancer Hospital (No.: 2024YNG03).
Author information
Authors and Affiliations
Contributions
CGS, SCT and ZHL supported and designed the study. QW, XWH, XFL, YS, and JLL collected, processed, and analysed the datasets. CXW, ZRJ, and XWH verified the data. QW and YSY wrote the manuscript. All of the authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Yunnan Provincial Cancer Hospital (Approval No. KYLX2024-060) and Fujian Cancer Hospital (Approval No. K2024-056-01), and written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Yu, Y., Wang, C. et al. Heterogeneity of tertiary lymphoid structures predicts the response to neoadjuvant therapy and immune microenvironment characteristics in triple-negative breast cancer. Br J Cancer 132, 295–310 (2025). https://doi.org/10.1038/s41416-024-02917-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-024-02917-y