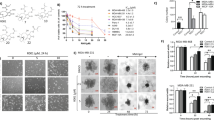
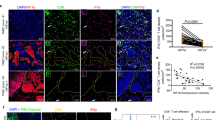
Abstract
Background
γδ T cells have emerged as pivotal regulators within the breast cancer tumour microenvironment (TME) and represent a promising therapeutic strategy for late-stage and metastatic breast cancer. In recent years, our research has focused on leveraging allogeneic Vγ9Vδ2 T cells as a novel approach to treat advanced cancers, including triple-negative breast cancer (TNBC). However, the varying clinical outcomes of this therapy have prompted us to investigate the diverse roles of γδ T cells within the TNBC microenvironment and to explore strategies for enhancing therapeutic efficacy through microenvironmental remodelling.
Methods
Data from TCGA, publicly available scRNA-seq datasets and a series of experiments including flow cytometry, atomic force microscopy, confocal laser scanning microscopy, mouse model and others were applied to examine the functional heterogeneity of γδ T cells in TNBC, non-TNBC, and healthy individuals.
Results
γδ T cells serve as predictive markers of better prognosis in breast cancer. In TNBC tumours, γδ T cells exhibited heightened expression of genes linked to both effector and inhibitory molecules, alongside a significant upregulation of glycolytic activity—patterns not observed in non-TNBC or normal breast tissues. Further analysis demonstrated that hypoxic conditions in the TNBC microenvironment likely contribute to these metabolic changes, leading to upregulation of inhibitory checkpoints and downregulation of effector functions in γδ T cells. Importantly, we showed that suppressing HIF-1 signalling using PX478 enhanced the antitumor efficacy of Vδ2+γδ T cell therapy in TNBC-bearing mice.
Discussion
This work underscores that remodelling the hypoxic TNBC microenvironment can restore the antitumor activity of Vγ9Vδ2 T cell therapy. Our findings offer a compelling new adjuvant strategy to improve the outcomes of Vγ9Vδ2 T cell-based therapies for advanced breast cancer treatment.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
269,00 € per year
only 11,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data are provided within the manuscript or supplementary information files.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–63.
Xia X, Cao G, Sun G, Zhu L, Tian Y Song Y et al. GLS1-mediated glutaminolysis unbridled by MALT1 protease promotes psoriasis pathogenesis. J Clin Investig. 2020;130:5180–96.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34.
Zagami P, Carey LA. Triple negative breast cancer: pitfalls and progress. NPJ Breast Cancer. 2022;8:95.
Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–8.
Silva-Santos B, Mensurado S, Coffelt SB. gammadelta T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19:392–404.
Hu Y, Hu Q, Li Y, Lu L, Xiang Z, Yin Z et al. γδ T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct Target Ther. 2023;8:434.
Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–42.
Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat Med. 2015;21:938–45.
Xu Y, Xiang Z, Alnaggar M, Kouakanou L, Li J, He J et al. Allogeneic Vgamma9Vdelta2 T-cell immunotherapy exhibits promising clinical safety and prolongs the survival of patients with late-stage lung or liver cancer. Cell Mol Immunol. 2021;18:427–39.
Alnaggar M, Xu Y, Li J, He J, Chen J, Li M et al. Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J Immunother Cancer. 2019;7:36.
Mensurado S, Blanco-Dominguez R, Silva-Santos B. The emerging roles of gammadelta T cells in cancer immunotherapy. Nat Rev Clin Oncol. 2023;20:178–91.
Janssen A, Villacorta Hidalgo J, Beringer DX, van Dooremalen S, Fernando F, van Diest E et al. gammadelta T-cell receptors derived from breast cancer-infiltrating T lymphocytes mediate antitumor reactivity. Cancer Immunol Res. 2020;8:530–43.
Wu Y, Kyle-Cezar F, Woolf RT, Naceur-Lombardelli C, Owen J, Biswas D et al. An innate-like Vdelta1(+) gammadelta T cell compartment in the human breast is associated with remission in triple-negative breast cancer. Sci Transl Med. 2019;11:eaax9364.
Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–8.
Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8:864.
Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27:334–48.
Ni C, Fang QQ, Chen WZ, Jiang JX, Jiang Z, Ye J et al. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73+gammadelta1 Treg cells. Signal Transduct Target Ther. 2020;5:41.
Buck MD, Sowell RT, Kaech SM, Pearce EL. Metabolic instruction of immunity. Cell. 2017;169:570–86.
Li Y, Wu Y, Hu Y. Metabolites in the tumor microenvironment reprogram functions of immune effector cells through epigenetic modifications. Front Immunol. 2021;12:641883.
Madden MZ, Rathmell JC. The complex integration of T-cell metabolism and immunotherapy. Cancer Discov. 2021;11:1636–43.
Lopes N, McIntyre C, Martin S, Raverdeau M, Sumaria N, Kohlgruber AC et al. Distinct metabolic programs established in the thymus control effector functions of gammadelta T cell subsets in tumor microenvironments. Nat Immunol. 2021;22:179–92.
Park JH, Kim HJ, Kim CW, Kim HC, Jung Y, Lee HS et al. Tumor hypoxia represses gammadelta T cell-mediated antitumor immunity against brain tumors. Nat Immunol. 2021;22:336–46.
Vignali PDA, DePeaux K, Watson MJ, Ye C, Ford BR, Lontos K et al. Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity. Nat Immunol. 2023;24:267–79.
Ma S, Zhao Y, Lee WC, Ong LT, Lee PL, Jiang Z et al. Hypoxia induces HIF1alpha-dependent epigenetic vulnerability in triple negative breast cancer to confer immune effector dysfunction and resistance to anti-PD-1 immunotherapy. Nat Commun. 2022;13:4118.
Bhandari V, Li CH, Bristow RG, Boutros PC, Consortium P. Divergent mutational processes distinguish hypoxic and normoxic tumours. Nat Commun. 2020;11:737.
McEwen GD, Wu Y, Tang M, Qi X, Xiao Z, Baker SM et al. Subcellular spectroscopic markers, topography and nanomechanics of human lung cancer and breast cancer cells examined by combined confocal Raman microspectroscopy and atomic force microscopy. Analyst. 2013;138:787–97.
Wu YZ, McEwen GD, Harihar S, Baker SM, DeWald DB, Zhou AH. BRMS1 expression alters the ultrastructural, biomechanical and biochemical properties of MDA-MB-435 human breast carcinoma cells: an AFM and Raman microspectroscopy study. Cancer Lett. 2010;293:82–91.
Hu Y, Hu Q, Li Y, Lu L, Xiang Z, Yin Z et al. gammadelta T cells: origin and fate, subsets, diseases and immunotherapy. Signal Transduct Target Ther. 2023;8:434.
Schmolka N, Serre K, Grosso AR, Rei M, Pennington DJ, Gomes AQ et al. Epigenetic and transcriptional signatures of stable versus plastic differentiation of proinflammatory gammadelta T cell subsets. Nat Immunol. 2013;14:1093–100.
Wu YZ, Lu HS, Cai JY, He XH, Hu Y, Zhao HX et al. Membrane surface nanostructures and adhesion property of T lymphocytes exploited by AFM. Nanoscale Res Lett. 2009;4:942–7.
Jung P, Zhou X, Iden S, Bischoff M, Qu B. T cell stiffness is enhanced upon formation of immunological synapse. Elife. 2021;10:e66643.
Ma VP, Hu Y, Kellner AV, Brockman JM, Velusamy A, Blanchard AT et al. The magnitude of LFA-1/ICAM-1 forces fine-tune TCR-triggered T cell activation. Sci Adv. 2022;8:eabg4485.
Chaudhuri O, Parekh SH, Fletcher DA. Reversible stress softening of actin networks. Nature. 2007;445:295–8.
Hosokawa H, Rothenberg EV. How transcription factors drive choice of the T cell fate. Nat Rev Immunol. 2021;21:162–76.
Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–61.
Wu H, Zhao X, Hochrein SM, Eckstein M, Gubert GF, Knopper K et al. Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1alpha-mediated glycolytic reprogramming. Nat Commun. 2023;14:6858.
Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70.
Taylor CT, Scholz CC. The effect of HIF on metabolism and immunity. Nat Rev Nephrol. 2022;18:573–87.
Tibes R, Falchook GS, Von Hoff DD, Weiss GJ, Lyengar T, Kurzrock R et al. Results from a phase I, dose-escalation study of PX-478, an orally available inhibitor of HIF-1α. J Clin Oncol. 2010;28:3076.
Acknowledgements
We also thank Dr. Qixin Chen and colleagues from the Pangu MedTech (Guangzhou) for their assistance with scRNA-seq data analysis.
Funding
This work is supported by the National Natural Science Foundation of China (82002787) and the Natural Science Foundation of Guangdong Province of China (2025A1515010628) (YH). YZW is supported by the National Natural Science Foundation of China (32270950), the Natural Science Foundation of Guangdong Province of China (2024A1515010551), the Startup Foundation of the Zhuhai People’s Hospital (YNXM20210305), and partially by the Key Program of the National Natural Science Foundation of China (32030036). XH is supported by the Science and Technology Projects in Guangzhou (202201020050), the Guangdong Medical Science and Technology Research Fund Project (A2023138), and the Beijing Science and Technology Innovation Medical Development Foundation (KC2023-JX-0270-03).
Author information
Authors and Affiliations
Contributions
Work supervision and design: YH and YZW. Experiments: YH, YYJ, YZW and QLH. Clinical samples and data collection: YH, YYJ, XH, DL, WHH and MGF. Data analysis and discussion: YH, YZW and YYJ. Mouse model: YYJ, QLH and WFW. Manuscript writing and revision: YH, YZW and YYJ. All authors approved the final version of manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The animal related experiment was approved by the Jinan University Laboratory Animal Ethics Committee (#20210712-28).
Consent for publication
All co-authors hereby grant their consent for the publication of our work, understanding that it may be shared publicly.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jing, Y., Wu, Y., Hu, Q. et al. Remodelling hypoxic TNBC microenvironment restores antitumor efficacy of Vγ9Vδ2 T cell therapy. Br J Cancer (2025). https://doi.org/10.1038/s41416-025-03045-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-025-03045-x