Abstract
Background
The association between statins and breast cancer-specific mortality and recurrence has been examined in several previous observational studies and meta-analyses. However, potentially important effect modifiers have not often been explored in previous meta-analyses. In this study, an updated systematic review and meta-analysis was undertaken to ascertain the association between statins and both breast cancer death (BCD) and breast cancer recurrence (BCR).
Methods
Articles were sourced from various databases up until the 13th of June 2024, and effect estimates were pooled using the random effects model. Subgroup analyses were conducted by the potential for immortal time bias (ITB), type of statin (lipophilic vs hydrophilic), estrogen receptor status (positive vs negative), stage (‘early’ vs ‘advanced’), and type of postdiagnostic use (‘new’ vs ‘prevalent’ user).
Results
Pooled results showed that there was a statistically significant protective association between statin use and both BCD (21 studies, hazard ratio = 0.81, 95% CI: 0.75–0.87) and BCR (20 studies, HR = 0.81, 95% CI: 0.74–0.89). Lipophilic statins were more protective than hydrophilic statins with BCD as the outcome, and there were suggestions of a more protective association in studies with ITB and in ER+ patients with BCR as the outcome. There was little evidence of effect modification by stage or type of postdiagnostic use.
Conclusion
In this meta-analysis, we observed that statin use, particularly lipophilic statin use, was associated with favourable outcomes for BCD and BCR.
Similar content being viewed by others
Background
Breast cancer is the most common cancer in women and the leading cause of female cancer mortality worldwide [1]. Comorbidities are common in patients with breast cancer [2], and there is a high and increasing prevalence of risk factors for both breast cancer and ischaemic heart disease among Western women [3,4,5]. As such, many patients with breast cancer use prescribed medications for cardiovascular conditions. Ascertaining the association between commonly used cardiovascular medications and breast cancer-specific mortality and recurrence is therefore warranted. Statins (3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors) are the most widely prescribed cholesterol lowering medications [6] and are used for both the primary and secondary prevention of cardiovascular disease [7].
Statins reduce cholesterol levels by inhibiting the rate limiting enzyme of the mevalonate pathway (HMGCR), which has been shown to be over expressed in breast cancer tumours [8, 9]. Statins have been found to exert pleiotropic effects, such as the induction of apoptosis, inhibition of proliferation, as well as expressing immunomodulatory properties [10,11,12]. In preclinical studies, statins have also reported to be associated with anti-neoplastic properties in animal models and breast cancer cell lines [13,14,15]. On the basis of this evidence, a number of observational studies have been carried out, and several meta-analyses have been published to summarise these studies [16,17,18,19,20,21,22,23,24]. Published between 2015 and 2023 and covering studies published up until the 1st of June 2023, most of these meta-analyses have shown a statistically significant protective association between the use of statins and the prognosis of breast cancer.
In the meta-analyses published to date, some of the more recent observational studies have not been included [25,26,27,28,29]. Some of the meta-analyses have also missed previously published observational studies, perhaps owing to insufficient and/or incomplete literature search strategies or different eligibility criteria being used. Further, there have not been any meta-analyses that have examined potentially important effect modifiers of the association such as the presence of immortal time bias (ITB), the stage of breast cancer (‘early’ vs ‘advanced’ stage), or the type of postdiagnostic statin use (‘new’ vs ‘prevalent’ user). ITB is a bias in which a spurious survival advantage is generally conferred to the user group in studies that count user time in a period where events could not occur by design [30]. For example, if a woman is dispensed a statin at a certain point in time after her diagnosis, it is not possible that she died between her diagnosis and the statin dispensing. Moreover, recent experimental and observational studies have indicated that statins may exert their effect on late-stage tumour progression/metastasis [13, 31,32,33], and knowing if the effect of statins is modified by stage would provide important information to clinicians when prescribing statins to breast cancer patients. Finally, it is widely acknowledged in the pharmacoepidemiological literature that drug initiators (i.e., ‘new’ users) will likely differ on important health characteristics relative to existing (or ‘prevalent’) users [34]. While the type of statin (lipophilic vs hydrophilic) and patients’ estrogen receptor (ER) status (positive vs negative) have been examined in subgroup analyses in some previous meta-analyses, we also wanted to assess these variables as potential effect modifiers in updated subgroup analyses. As such, the objective of the current paper was to perform an updated systematic review and meta-analysis examining the association between statins and both breast cancer death (BCD) and breast cancer recurrence (BCR), and also examine whether the associations differ across potentially important subgroups.
Methods
Literature search/data sources
PubMed, Embase, Medline, Web of Science, Google Scholar, and the Cochrane Library databases were searched using the keywords [statin, hydroxymethylglutaryl-CoA reductase inhibitor, HMG-CoA reductase inhibitor, cholesterol lowering, simvastatin, atorvastatin, pravastatin, lovastatin, fluvastatin, pitavastatin, breast cancer, breast carcinoma, cancer, carcinoma, recurrence, metastasis, outcome, death, prognosis, survival, mortality, proliferation] in combinations of ‘AND’ or ‘OR’. There was no restriction placed on the earliest date of publication, but articles were only selected for review if they analysed human breast cancer patients and were written in English. Further articles were sourced from scanning the reference lists of individual observational studies, systematic reviews, and meta-analyses found using the aforementioned search criteria. Literature was sourced up until the 13th of June 2024.
Study selection/eligibility criteria
Studies were eligible for inclusion if they met the following criteria: (1) Included women with breast cancer as the cohort of interest; (2) Analysed data from cohort studies, case-control studies, or randomised controlled trials (including retrospective analyses of already published RCTs); (3) Assessed statin use as the exposure of interest (with no minimum dose requirement); (4) Reported BCD or BCR (or both) as outcomes of interest; (5) Reported 95% confidence intervals or standard errors for the associations of interest; and (6) Were published as full length articles. We did not consider laboratory experiments, case reports, ecological studies, conference abstracts, or editorials for inclusion in this meta-analysis.
Data extraction
The titles and abstracts of each individual study were first examined to assess their eligibility, and relevant information from each study was then independently extracted by two authors (OS and STT). We subsequently liaised with each other to resolve any disagreements and come to a consensus. Information extracted included the first author and year of study, country of study, the number of women under study, study design (prospective cohort vs retrospective cohort; prospective cohort studies were those that actively enroled a cohort of patients and followed them up over time, while retrospective cohort studies were studies that retrospectively analysed already recorded/collected data), patient characteristics (such as estrogen receptor status, cancer stage, and age), exposure definition (pre or postdiagnosis), type of postdiagnostic use (‘new’ vs ‘prevalent’ user; ‘new’ postdiagnostic users were defined as statin users who did not have a prescription/dispensing in a specified time period prior to diagnosis, while ‘prevalent’ postdiagnostic users were defined as statin users who could have also used statins prior to diagnosis), the source of exposure data, median or mean follow up time, the potential for ITB (studies were judged to be susceptible to ITB when they used a ‘time fixed’ postdiagnosis definition of medication use; these studies erroneously counted the time between a woman’s cancer diagnosis and first statin prescription/dispensing as user time instead of correctly classifying it as nonuser time), covariates adjusted for, the types of statins used by women (e.g., all statins, lipophilic statins, hydrophilic statins, or a combination), and the reference group used (e.g. statin users vs all statin nonusers or statin users vs a different comparison group). When studies used a stepwise approach for covariate adjustment, the fully adjusted HR was taken.
Statistical analysis
The main analysis assessed the association between statin use at any time and breast cancer-specific mortality and recurrence, pooling the data from all included studies. When studies reported risk estimates for statins used both before and after breast cancer diagnosis for the same cohort, only the risk estimates for postdiagnosis use were taken (to avoid sample overlap), as it can be considered that postdiagnosis use of medications over the course of breast cancer therapy is the more clinically relevant exposure period. Risk estimates and 95% confidence intervals were transformed onto the log scale, as recommended [35]. To pool risk estimates into a summary estimate, the inverse variance method with random effects model was used. We used a random effects model because it seems plausible that the effect of statins could vary from study to study due to factors other than sampling variability [36]. In the paper by Li and others [37], the HRs for the associations between statin use and BCR in all patients and statin use and BCR in ER+ and ER- patients were initially stratified by length of statin use (<3 years, 3–5 years, and 5+ years), and no overall HR was reported. These HRs were first pooled using a random-effects model to derive an overall summary estimate for any statin use, and the resulting pooled HRs were then included in this meta-analysis. A similar method was used to pool results for the associations between statin use and BCR in all patients and statin use and BCR in ER- patients in the paper by Sim and colleagues [38]. The same method was again employed to derive overall summary estimates for lipophilic statin use in the papers by Murtola et al. and Nowakowska et al. [39, 40] and in ‘early’ stage (nonmetastatic) patients in the papers by Lofling and colleagues and Murto et al. [25, 26]. Finally, the same method was again used to pool results in ER+ and ER- patients for both BCD and BCR in the paper by Guo and others [29] and to derive an overall summary estimate for BCR in the paper by Dumas and colleagues [27]. The Higgins I2 statistic was computed for each pooled estimate to ascertain the amount of heterogeneity present between studies, and an I2 statistic of >50% indicated that there was a significant amount of heterogeneity [41].
To explore reasons for heterogeneity between studies and to assess the impact of different potential modifying variables on the associations of interest, subgroup analyses [42] were carried out by the potential for ITB (yes vs no), the type of statin used (lipophilic vs hydrophilic), ER status (positive vs negative), the stage of breast cancer (‘early’ vs ‘advanced’), and the type of postdiagnostic use (‘new’ vs ‘prevalent’ user). The intention of the subgroup analysis by stage was to analyse metastatic (‘advanced’ stage) patients vs all others, however due to the relative dearth of studies that stratified their analyses by stage, any study that did so was included in this subgroup analysis instead of studies that exclusively examined metastatic patients vs others. All studies examining BCR as an outcome of interest in this meta-analysis only included ‘early’ stage (nonmetastatic) patients, and this subgroup analysis was therefore restricted to BCD only. Because of the bias inherent in studies judged to be susceptible to ITB [30], the subgroup analyses by type of statin, ER status, stage of breast cancer, and type of postdiagnostic use were restricted to studies judged not to be susceptible to ITB. Furthermore, because studies that assess medication use prior to cancer diagnosis are inherently protected from ITB, we carried out an additional subgroup analysis by ITB in studies that exclusively assessed medication use after cancer diagnosis. To formally test for differences between subgroups, a random effects meta-regression was used [43]. Lastly, funnel plots [44] were generated, and Egger’s regression tests [45] were performed to assess the presence of any potential publication bias. If publication bias was judged to be present, trim and fill analysis [46] was conducted to assess any potential change(s) in the summary estimate(s). All reported p values are two-sided and were considered statistically significant if p < 0.05. All analyses were conducted in STATA 17.0 (StataCorp, College Station, TX).
Results
Literature search
A total of 638 studies (after removing duplicates) were retrieved from the selected databases during the literature search and included for initial screening (Fig. 1). Two hundred and two reports were assessed in full, and after making various exclusions, 34 studies were deemed to meet the inclusion criteria and were included in this meta-analysis [25,26,27,28,29, 31, 37,38,39,40, 47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70]. The detailed characteristics of these 34 studies are shown in Table 1.
Study characteristics
Overall, this meta-analysis included 34 studies [25,26,27,28,29, 31, 37,38,39,40, 47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] with a combined total of 689,990 women with breast cancer. Twenty-one of these studies assessed BCD as an outcome, while twenty assessed BCR as an outcome (seven of these studies assessed both outcomes). Twenty of the 34 studies were conducted in Europe (including the UK), ten in North America, three from the Asia/Oceania region, and one was from South America. Twenty-nine were designed as retrospective cohort studies and five were prospective cohort studies. All studies adjusted for covariates, and every study (except for two [28, 70]) adjusted for age at the very least. In general, a range of confounders were usually adjusted for, including demographic variables, breast cancer clinical variables, comorbidities, and concomitant medication usage. For example, potentially important confounders such as cancer stage, comorbidities (or an index of the severity of comorbid conditions), and concomitant medication use (excluding hormonal therapy) were adjusted for in 25/34, 20/34, and 16/34 studies, respectively. The mean or median follow up time across the 34 studies ranged from 2.0 to 11.5 years, with sixteen studies reporting a mean/median follow up time of 0–5 years, fourteen reporting a mean/median follow up time of 5–10 years, and one reporting a mean/median follow up time of 10+ years. Follow-up time was not reported in three studies. Twenty-seven studies analysed statin use after the diagnosis of breast cancer (of these, eight included ‘new’ users only, while 19 analysed ‘prevalent’ users), five considered prediagnosis statin use as the exposure of interest, and two analysed both periods concurrently. Twenty-two studies ascertained medication use through prescription records, eight through medical records, and four through self-reported patient data. Twenty-seven studies were judged not to be susceptible to ITB, while seven were. There were fourteen studies that stratified statin use by type (lipophilic vs hydrophilic), one that analysed only lipophilic statins in a sensitivity analysis, while nineteen did not stratify by the type of statin. Eleven studies stratified cancers by their ER status (positive vs negative), two included only ER- patients, one analysed only ER+ patients in a sensitivity analysis, while twenty did not stratify by ER status. Finally, there were five studies that stratified by cancer stage (‘early’ vs ‘advanced’), twenty-one that included only ‘early’ stage (nonmetastatic) patients, while eight did not stratify by cancer stage.
Association between statin use and breast cancer related deaths
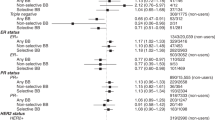
Overall, 21 studies were included in the meta-analysis examining the association between statin use and breast cancer-specific mortality. Pooled results with a random effects model showed that there was a statistically significant protective association between statin use and BCD (HR = 0.81, 95% CI: 0.75–0.87, p < 0.001; Fig. 2a). There was significant heterogeneity between these studies (I2 = 69.44%).
Subgroup analyses showed that the type of statin used may affect the outcome. In the studies that examined lipophilic statin use, the results indicated that these statins were associated with a statistically significant protective association on BCD (HR = 0.75, 95% CI: 0.62–0.91, p = 0.003, Table 2 and Supplementary Fig. 1), and the association with hydrophilic statins also indicated a statistically significant protective association, but smaller in magnitude (HR = 0.93, 95% CI: 0.89–0.97, p = 0.001; p for subgroup difference = 0.05). Other analyses revealed that stratifying the association by other potential modifiers (presence of ITB, estrogen receptor status, stage, and type of postdiagnostic use) did not significantly change the results (p values for subgroup differences all > 0.05; Table 2, Supplementary Fig. 1 and Supplementary Table 1). Furthermore, in studies that exclusively assessed medication use after cancer diagnosis, there was no difference found when stratifying the association by the presence of ITB.
Association between statin use and breast cancer recurrence
Overall, 20 studies were included in the meta-analysis examining the association between statin use and breast cancer recurrence. Pooled results with a random effects model showed that there was a statistically significant protective association between statin use and BCR (HR = 0.81, 95% CI: 0.74-0.89, p < 0.001; Fig. 2b). There was significant heterogeneity between these studies (I2 = 51.77%).
Subgroup analyses revealed that stratifying the association by potential modifiers (presence of ITB, type of statin, estrogen receptor status, and type of postdiagnostic use) did not significantly change the results (p values for subgroup differences all > 0.05; Table 2, Supplementary Fig. 2, and Supplementary Table 1). The pooled HRs were lower for studies judged to be susceptible to ITB relative to studies judged not to be susceptible to ITB (in all studies as well as in studies that exclusively assessed medication use after cancer diagnosis) and for patients with ER+ cancers relative to patients with ER- cancers, however these differences were not statistically significant.
Publication bias
There was little evidence of publication bias or small study effects (p value for small study effects = 0.94) for studies examining the association between statin use and BCD (Fig. 3a). Conversely, the funnel plot for the association between statin use and BCR showed clear evidence of asymmetry (Fig. 3b). In this plot, there was asymmetry in terms of the number of studies on either side of the average effect size, with the plot having more studies on the left-hand side (meaning that there were more studies that showed a lower HR than the overall average effect size relative to studies that showed a higher HR). Further, there was clear evidence of small study effects in this plot, with a big cluster of small studies with large effect sizes toward the bottom left of the plot. The result of Egger’s regression test was in concordance with a visual inspection of the funnel plot (p value for small study effects = 0.002). When excluding studies judged to be susceptible to ITB and performing trim and fill analysis [46] on studies assessing BCR as an outcome, there was still a statistically significant protective association shown between statin use and BCR (HR = 0.86, 95% CI: 0.79–0.95; Supplementary Fig. 3).
Discussion
In this systematic review and meta-analysis, we found a statistically significant protective association between statin use and both BCD and BCR, with a 19% reduction in risk for both outcomes. Our findings are consistent with almost every other meta-analysis examining the association between statin use and breast cancer prognosis [16,17,18,19,20,21,22,23,24]. Every previous meta-analysis that has examined BCR as an outcome has found a statistically significant protective association with statin use, and all but one previous meta-analysis (which was published in 2016) [18] has found a statistically significant protective association between statin use and BCD. In the 2016 meta-analysis by Manthravadi and colleagues, the association between statin use and BCD was bordering on statistical significance (HR = 0.70, 95% CI: 0.46–1.06, p = 0.09).
Immortal time bias (ITB) is a bias introduced in pharmacoepidemiological studies in which a survival advantage is generally conferred to the user group by way of misattributing user time over a period where events could not occur by design [30]. For example, if a postdiagnostic exposure period is used and a binary yes/no indicator is used to model medication use (i.e., a time fixed approach), the time between cancer diagnosis and the first medication dispensing is ‘immortal’ because the patient needed to have survived this period to be dispensed a medication. Lévesque and colleagues showed that medications with no biological basis for doing so (e.g., NSAIDS) could be made to show a decreased risk of diabetes progression by modelling medication use through a time fixed approach [71]. The subgroup analyses by ITB in this meta-analysis did not show the pattern we were expecting, with both outcomes showing no statistically significant difference between studies judged to be susceptible to ITB relative to studies judged not to be susceptible to ITB (for both the ‘main’ ITB analysis as well as the ITB analysis in which we exclusively considered postdiagnostic use). However, the difference between groups with BCR as the outcome was approaching statistical significance in both ITB analyses. To our knowledge, this is the first meta-analysis in this area to examine ITB as a potential effect modifier. When considering BCD as the outcome in the ‘main’ ITB analysis, there were only three studies that were judged to be susceptible to ITB. Further, some studies that were not judged to be susceptible to ITB, such as those by Murtola and colleagues [39], Sakellakis and colleagues [59], and Takada et al. [70] all showed very strong protective associations (i.e., a very low HR) between statin use and BCD/BCR. The HRs derived from these studies could be considered outliers, and there were no obvious reasons to explain the very low HRs derived in these studies.
In the subgroup analysis by type of statin, there was evidence of lipophilic statins being more efficacious than hydrophilic statins with BCD as the outcome, however, there was no effect modification shown with BCR as the outcome. The result with BCD is consistent with preclinical studies, which have indicated that only lipophilic statins have anti-proliferative effects on breast cancer cells [14, 72, 73]. Several previous meta-analyses have also examined the hydrophilicity of statins as a potential effect modifier, with two showing no evidence of effect modification for either outcome [18, 20], one also showing a more protective association for lipophilic statins with BCD as the outcome [19], and a recent meta-analysis by Jaiswal and colleagues showing a more protective association for lipophilic statins with BCR as the outcome [23]. The differing results by type of statin with BCR as the outcome between the meta-analysis by Jaiswal and others and the current meta-analysis can likely be explained by the additional inclusion of the studies by Brewer et al. [52], Dumas and others [27], and Guo et al. [29] in the current meta-analysis, which all showed a slightly lower HR (statistically non-significant differences) for hydrophilic statin users.
There have been hypotheses that statins may be more efficacious in ER+ breast cancers relative to ER- cancers. The more protective effect in ER+ tumours is thought to result from statins lowering levels of cholesterol metabolite 27-hydroxycholesterol (27HC), a selective estrogen receptor modulator that can regulate ER-dependent tumour growth [74,75,76]. The subgroup analysis by ER status with BCD as the outcome did not show any effect modification; however, the same analysis with BCR as the outcome was bordering on statistical significance, with a strong suggestion that statins were only protective in ER+ cancers. The number of studies available for this subgroup analysis with BCR as the outcome was small, and more studies would be desirable. One previous meta-analysis has also examined ER status as a potential effect modifier, and it found no effect modification for either outcome [21]. However, this meta-analysis erroneously included the study by Desai and colleagues [56] that examined the association between statins and late-stage breast cancer (i.e., incidence instead of prognosis), incorrectly classified the study by Ahern and others [49] as examining BCD instead of BCR, and also included a study by Borgquist et al. that examined all cholesterol lowering medications instead of statins exclusively [77]. The studies by Li et al. [37] and Shaitelman et al. [62] were also excluded from this subgroup analysis in the current meta-analysis due to them being judged to be susceptible to ITB.
Recent experimental data has shown that atorvastatin treatment significantly reduced the metastatic spread of breast cancer to the liver and lung, but had little impact on primary tumour cells [13]. A recent observational study also showed that statins were more efficacious in preventing distant metastasis than locoregional recurrence [31]. These findings suggest that statins may be more protective in advanced-stage patients relative to early-stage patients. To our knowledge, this is the first meta-analysis in this area to examine breast cancer stage as a potential effect modifier, and we were unable to identify any evidence of effect modification. It is worth mentioning that the number of studies in the ‘advanced’ stage arm was small, as well as the fact that this subgroup analysis included the studies by Murtola and others [39] and Murto et al. [26] which both showed a more protective association (statistically significant difference in Murto et al.) in ‘early’ stage patients.
The final subgroup analysis we carried out was by type of postdiagnostic use (‘new’ vs ‘prevalent’ user). We were unable to identify any evidence of effect modification for either outcome in this analysis. To the best of our knowledge, the type of postdiagnostic use has not been examined as a potential effect modifier in any previous meta-analysis. If statins are ever to be used in an adjuvant setting for breast cancer, it is important to ascertain if the protective effect applies to new drug initiators, as it only possible to prospectively prescribe medications to women with a recent diagnosis.
There was a significant amount of between-study heterogeneity present for both outcomes in this meta-analysis, even in many of the individual arms of the subgroup analyses. As shown in Table 1, different studies were often conducted over different time periods and in different countries, inevitably meaning that statins were often used in different clinical contexts. There was also little uniformity regarding the characteristics of women enroled or analysed, with a range of different cancer stages and ages studied. Similarly, the covariates adjusted for varied widely across studies, with some studies making comprehensive adjustments for a range of covariates, while other studies likely lacked access to a similar range of potential confounders. Finally, postdiagnosis statin use may mean use in the first year after diagnosis only or use any time after diagnosis. All of these factors likely play a role in influencing any effect estimate derived, especially when >1 of these factors differ from study to study.
We found clear evidence of publication bias and small study effects with BCR as the outcome, with evidence of asymmetry in the funnel plot and a cluster of small studies with large and protective effect sizes. As such, removing publication bias would attenuate the overall HR towards the null (1.0). However, even after excluding studies judged to be susceptible to ITB and ‘correcting’ for publication bias through the trim and fill method [46], there was still a statistically significant protective association shown between statin use and BCR (Supplementary Fig. 1).
The main strength of this meta-analysis is the large number of observational studies included and the resulting total number of women with breast cancer analysed (689,990). As evidenced by Supplementary Table 2, some of the meta-analyses published to date have missed previously published observational studies, while this review included every study in the table. Furthermore, we studied both BCD and BCR as outcomes, and the consistency in the results between these outcomes gives us confidence in the interpretation of the meta-analysis. We also conducted subgroup analyses by ITB, type of statin used, ER status, and breast cancer stage, which were all subgroups we were interested in a priori with legitimate methodological or clinical justifications for analysing. Finally, although we did not carry out a formal quality assessment of the studies included in this meta-analysis, ITB is a strong indicator of methodological quality for pharmacoepidemiological studies.
This study is not without its limitations. As mentioned above, there was significant between-study heterogeneity for both outcomes, and as a result, it is difficult to determine if the pooled effect estimates derived are driven by statin use exclusively, extraneous factors such as the study period and/or other modifying factors between studies, or a combination of these. For example, some studies were unable to adjust for indications for statin treatment (cardiovascular disease and/or cholesterol levels), and as such, there is likely to be some residual confounding by indication in these studies. Furthermore, even though a strong effort was made to correctly classify studies in terms of their risk of ITB (including emailing authors to clarify their statin exposure definition), it is possible that some studies were incorrectly classified. Moreover, not every study that stratified by stage did so in a uniform manner, and there was one study in the ‘advanced’ stage subgroup that included patients with stage 3 cancer [57]. Even though evidence suggests that statins may exert a more efficacious effect in postmenopausal women relative to premenopausal women [51, 69, 78, 79], we were unable to conduct a subgroup analysis by menopausal status due to how rarely this was reported. Finally, none of the individual studies included in this review were able to ascertain the adherence of statin use among women who were prescribed/dispensed statins. Such non-adherence would likely attenuate any effect estimates derived toward the null [80], however, it is not possible to quantify the extent of this attenuation without knowing the proportion of non-adherence.
In conclusion, we observed an association between statin use and favourable outcomes for BCD and BCR in this systematic review and meta-analysis. We showed that lipophilic statins may be more efficacious than hydrophilic statins with BCD as the outcome, and there were suggestions that studies judged to be susceptible to ITB and ER+ patients had a lower HR than their respective counterparts with BCR as the outcome. There was little evidence of effect modification by the stage of breast cancer or type of postdiagnostic use. However, there was a significant amount of between-study heterogeneity present for both outcomes, and evidence of publication bias with BCR as the outcome. Further research is warranted in patient subgroups defined by estrogen receptor status and stage to ascertain a targeted population of breast cancer patients that may benefit from statin therapy in the adjuvant setting.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Land LH, Dalton SO, Jensen MB, Ewertz M. Influence of comorbidity on the effect of adjuvant treatment and age in patients with early-stage breast cancer. Br J Cancer. 2012;107:1901–7.
Mehta LS, Watson KE, Barac A, Beckie TM, Bittner V, Cruz-Flores S, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation 2018;137:e30–e66.
Ministry of Health. Annual update of key results 2018/19: New Zealand Health Survey [Internet]. 2019 [cited 2024 Apr 7]. Available from: https://www.health.govt.nz/publication/annual-update-key-results-2018-19-new-zealand-health-survey.
Ford ES, Li C, Zhao G, Pearson WS, Tsai J, Greenlund KJ. Trends in low-risk lifestyle factors among adults in the United States: findings from the Behavioral Risk Factor Surveillance System 1996–2007. Prev Med. 2010;51:403–7.
Feingold KR. Triglyceride lowering drugs. In: Feingold K, Anawalt B, Blackman M, editors. South Dartmouth, MA: Endotext; 2021.
Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78.
Ehmsen S, Pedersen MH, Wang G, Terp MG, Arslanagic A, Hood BL, et al. Increased cholesterol biosynthesis is a key characteristic of breast cancer stem cells influencing patient outcome. Cell Rep. 2019;27:3927–38.
Borgquist S, Djerbi S, Ponten F, Anagnostaki L, Goldman M, Gaber A, et al. HMG-CoA reductase expression in breast cancer is associated with a less aggressive phenotype and influenced by anthropometric factors. Int J Cancer. 2008;123:1146–53.
Beckwitt CH, Brufsky A, Oltvai ZN, Wells A. Statin drugs to reduce breast cancer recurrence and mortality. Breast Cancer Res. 2018;20:144.
Blanco-Colio LM, Tunon J, Martin-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23.
Bellosta S, Ferri N, Bernini F, Paoletti R, Corsini A. Non-lipid-related effects of statins. Ann Med. 2000;32:164–76.
Beckwitt CH, Clark AM, Ma B, Whaley D, Oltvai ZN, Wells A. Statins attenuate outgrowth of breast cancer metastases. Br J Cancer. 2018;119:1094–105.
Ghosh-Choudhury N, Mandal CC, Ghosh-Choudhury N, Choudhury GG. Simvastatin induces derepression of PTEN expression via NFκB to inhibit breast cancer cell growth. Cell Signal. 2010;22:749–58.
Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66:8707–14.
Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41:554–67.
Mansourian M, Haghjooy-Javanmard S, Eshraghi A, Vaseghi G, Hayatshahi A, Thomas J. Statins use and risk of breast cancer recurrence and death: a systematic review and meta-analysis of observational studies. J Pharm Pharm Sci. 2016;19:72–81.
Manthravadi S, Shrestha A, Madhusudhana S. Impact of statin use on cancer recurrence and mortality in breast cancer: a systematic review and meta-analysis. Int J Cancer. 2016;139:1281–8.
Liu B, Yi Z, Guan X, Zeng YX, Ma F. The relationship between statins and breast cancer prognosis varies by statin type and exposure time: a meta-analysis. Breast Cancer Res Treat. 2017;164:1–11.
Lv H, Shi D, Fei M, Chen Y, Xie F, Wang Z, et al. Association between statin use and prognosis of breast cancer: a meta-analysis of cohort studies. Front Oncol. 2020;10:556243.
Xu WH, Zhou YH. The relationship between post-diagnostic statin usage and breast cancer prognosis varies by hormone receptor phenotype: a systemic review and meta-analysis. Arch Gynecol Obstet. 2021;304:1315–21.
Zhao G, Ji Y, Ye Q, Ye X, Wo G, Chen X, et al. Effect of statins use on risk and prognosis of breast cancer: a meta-analysis. Anticancer Drugs. 2022;33:e507–e18.
Jaiswal V, Agrawal V, Ang SP, Saleeb M, Ishak A, Hameed M, et al. Post-diagnostic statin use and its association with cancer recurrence and mortality in breast cancer patients: a systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2023;9:731–40
Jia X, Lu Y, Xu Z, Mu Q. Impact of statin use on breast cancer recurrence and mortality before and after diagnosis: a systematic review and meta-analysis. Front Oncol. 2023;13:1256747.
Löfling LL, Stoer NC, Andreassen BK, Ursin G, Botteri E. Low-dose aspirin, statins, and metformin and survival in patients with breast cancers: a Norwegian population-based cohort study. Breast Cancer Res. 2023;25:101.
Murto MO, Simolin N, Arponen O, Siltari A, Artama M, Visvanathan K, et al. Statin use, cholesterol level, and mortality among females with breast cancer. JAMA Netw Open. 2023;6:e2343861–e.
Dumas E, Grandal Rejo B, Gougis P, Houzard S, Abécassis J, Jochum F, et al. Concomitant medication, comorbidity and survival in patients with breast cancer. Nat Commun. 2024;15:2966.
Giorello MB, Marks MP, Osinalde TM, del Rosario Padin M, Wernicke A, Calvo JC, et al. Post-surgery statin use contributes to favorable outcomes in patients with early breast cancer. Cancer Epidemiol. 2024;90:102573.
Guo H, Malone KE, Heckbert SR, Li CI. Statin use and risks of breast cancer recurrence and mortality. cancer. 2024;130:3106–14.
Suissa S. Immortal time bias in pharmacoepidemiology. Am J Epidemiol. 2008;167:492–9.
Inasu M, Feldt M, Jernstrom H, Borgquist S, Harborg S. Statin use and patterns of breast cancer recurrence in the Malmö diet and cancer study. Breast 2022;61:123–8.
Warita K, Warita T, Beckwitt CH, Schurdak ME, Vazquez A, Wells A, et al. Statin-induced mevalonate pathway inhibition attenuates the growth of mesenchymal-like cancer cells that lack functional E-cadherin mediated cell cohesion. Sci Rep. 2014;4:7593.
Koohestanimobarhan S, Salami S, Imeni V, Mohammadi Z, Bayat O. Lipophilic statins antagonistically alter the major epithelial-to-mesenchymal transition signaling pathways in breast cancer stem-like cells via inhibition of the mevalonate pathway. J Cell Biochem. 2019;120:2515–31.
Schneeweiss S, Patrick AR, Sturmer T, Brookhart MA, Avorn J, Maclure M, et al. Increasing levels of restriction in pharmacoepidemiologic database studies of elderly and comparison with randomized trial results. Med. Care. 2007;45:S131–42.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142.
Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111.
Li YR, Ro V, Steel L, Carrigan E, Nguyen J, Williams A, et al. Impact of long-term lipid-lowering therapy on clinical outcomes in breast cancer. Breast Cancer Res Treat. 2019;176:669–77.
Sim Y, Lim C, Phyu N, Tan KTB, Chew LST, Wong CY, et al. The impact of statin use and breast cancer recurrence - a retrospective study in Singapore. Front Oncol. 2022;12:835320.
Murtola TJ, Visvanathan K, Artama M, Vainio H, Pukkala E. Statin use and breast cancer survival: a nationwide cohort study from Finland. PLoS ONE. 2014;9:e110231.
Nowakowska MK, Lei X, Thompson MT, Shaitelman SF, Wehner MR, Woodward WA, et al. Association of statin use with clinical outcomes in patients with triple-negative breast cancer. Cancer 2021;127:4142–50.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60.
Borenstein M, Higgins JP. Meta-analysis and subgroups. Prev Sci. 2013;14:134–43.
Baker WL, White CM, Cappelleri JC, Kluger J, Coleman CI, Health Outcomes P, et al. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pr. 2009;63:1426–34.
Sterne JA, Becker BJ, Egger M. The funnel plot. In: Rothstein HR, Sutton AJ, Borenstein M, eds. Publication bias in meta‐analysis: prevention, assessment and adjustments: John Wiley & Sons; 2005. p. 76–98.
Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63.
Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res Treat. 2008;109:573–9.
Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, et al. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Investig. 2011;29:585–93.
Ahern TP, Pedersen L, Tarp M, Cronin-Fenton DP, Garne JP, Silliman RA, et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J Natl Cancer Inst. 2011;103:1461–8.
Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–802.
Nickels S, Vrieling A, Seibold P, Heinz J, Obi N, Flesch-Janys D, et al. Mortality and recurrence risk in relation to the use of lipid-lowering drugs in a prospective breast cancer patient cohort. PLoS ONE. 2013;8:e75088.
Brewer TM, Masuda H, Liu DD, Shen Y, Liu P, Iwamoto T, et al. Statin use in primary inflammatory breast cancer: a cohort study. Br J Cancer. 2013;109:318–24.
Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, et al. Therapeutic effect of beta-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat. 2013;140:567–75.
Lacerda L, Reddy JP, Liu D, Larson R, Li L, Masuda H, et al. Simvastatin radiosensitizes differentiated and stem-like breast cancer cell lines and is associated with improved local control in inflammatory breast cancer patients treated with postmastectomy radiation. Stem Cells Transl Med. 2014;3:849–56.
Boudreau DM, Yu O, Chubak J, Wirtz HS, Bowles EJ, Fujii M, et al. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res Treat. 2014;144:405–16.
Desai P, Lehman A, Chlebowski RT, Kwan ML, Arun M, Manson JE, et al. Statins and breast cancer stage and mortality in the Women’s Health Initiative. Cancer Causes Control. 2015;26:529–39.
Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin use after diagnosis of breast cancer and survival: a population-based cohort study. Epidemiology 2015;26:68–78.
Smith A, Murphy L, Sharp L, O’Connor D, Gallagher WM, Bennett K, et al. De novo post-diagnosis statin use, breast cancer-specific and overall mortality in women with stage I-III breast cancer. Br J Cancer. 2016;115:592–8.
Sakellakis M, Akinosoglou K, Kostaki A, Spyropoulou D, Koutras A. Statins and risk of breast cancer recurrence. Breast Cancer. 2016;8:199–205.
Mc Menamin UC, Murray LJ, Hughes CM, Cardwell CR. Statin use and breast cancer survival: a nationwide cohort study in Scotland. BMC Cancer. 2016;16:600.
Smith A, Murphy L, Zgaga L, Barron TI, Bennett K. Pre-diagnostic statin use, lymph node status and mortality in women with stages I-III breast cancer. Br J Cancer. 2017;117:588–96.
Shaitelman SF, Stauder MC, Allen P, Reddy S, Lakoski S, Atkinson B, et al. Impact of statin use on outcomes in triple negative breast cancer. J Cancer. 2017;8:2026–32.
Haukka J, Niskanen L, Auvinen A. Risk of cause-specific death in individuals with cancer-modifying role diabetes, statins and metformin. Int J Cancer. 2017;141:2437–49.
Tryggvadottir H, Huzell L, Gustbee E, Simonsson M, Markkula A, Jirstrom K, et al. Interactions between ABCB1 genotype and preoperative statin use impact clinical outcomes among breast cancer patients. Front Oncol. 2018;8:428.
Borgquist S, Broberg P, Tojjar J, Olsson H. Statin use and breast cancer survival - a Swedish nationwide study. BMC Cancer. 2019;19:54.
Hosio M, Urpilainen E, Hautakoski A, Marttila M, Arffman M, Sund R, et al. Survival after breast cancer in women with type 2 diabetes using antidiabetic medication and statins: a retrospective cohort study. Acta Oncol. 2020;59:1110–7.
Harborg S, Heide-Jorgensen U, Ahern TP, Ewertz M, Cronin-Fenton D, Borgquist S. Statin use and breast cancer recurrence in postmenopausal women treated with adjuvant aromatase inhibitors: a Danish population-based cohort study. Breast Cancer Res Treat. 2020;183:153–60.
Bjarnadottir O, Feldt M, Inasu M, Bendahl PO, Elebro K, Kimbung S, et al. Statin use, HMGCR expression, and breast cancer survival - the Malmö diet and cancer study. Sci Rep. 2020;10:558.
Scott OW, Tin Tin S, Harborg S, Kuper-Hommel MJJ, Lawrenson R, Elwood JM. Post-diagnostic statin use and breast cancer-specific mortality: a population-based cohort study. Breast Cancer Res Treat. 2023;199:195–206.
Takada K, Kashiwagi S, Iimori N, Kouhashi R, Yabumoto A, Goto W, et al. Impact of oral statin therapy on clinical outcomes in patients with cT1 breast cancer. BMC Cancer. 2023;23:224.
Levesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087.
Hamelin BA, Turgeon J. Hydrophilicity/lipophilicity: relevance for the pharmacology and clinical effects of HMG-CoA reductase inhibitors. Trends Pharm Sci. 1998;19:26–37.
Matusewicz L, Meissner J, Toporkiewicz M, Sikorski AF. The effect of statins on cancer cells-review. Tumour Biol. 2015;36:4889–904.
McDonnell DP, Chang C-Y, Nelson E. The estrogen receptor as a mediator of the pathological actions of cholesterol in breast cancer. Climacteric 2014;17:60–5.
Kimbung S, Lettiero B, Feldt M, Bosch A, Borgquist S. High expression of cholesterol biosynthesis genes is associated with resistance to statin treatment and inferior survival in breast cancer. Oncotarget 2016;7:59640–51.
Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, et al. 27-hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013;342:1094–8.
Borgquist S, Giobbie-Hurder A, Ahern TP, Garber JE, Colleoni M, Láng I, et al. Cholesterol, cholesterol-lowering medication use, and breast cancer outcome in the BIG 1-98 study. J Clin Oncol. 2017;35:1179–88.
Zhao H, Zhou L, Shangguan AJ, Bulun SE. Aromatase expression and regulation in breast and endometrial cancer. J Mol Endocrinol. 2016;57:R19–33.
Brodie AM, Njar VC. Aromatase inhibitors and their application in breast cancer treatment. Steroids 2000;65:171–9.
Hernan MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9:48–55.
Acknowledgements
We would like to thank the Auckland Medical Research Foundation for providing funding to carry out this study.
Funding
Oliver Scott was supported by an Auckland Medical Research Foundation doctoral scholarship (Ref: 1217004). This project was also supported by an Auckland Medical Research Foundation project grant (Ref: 1118017). Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
OWS substantially contributed to the conception and design of the study and interpreted the data alongside STT, AC, and JME. OWS led the analysis and wrote the manuscript. All authors critically revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scott, O.W., Tin Tin, S., Cavadino, A. et al. Statin use and breast cancer-specific mortality and recurrence: a systematic review and meta-analysis including the role of immortal time bias and tumour characteristics. Br J Cancer (2025). https://doi.org/10.1038/s41416-025-03070-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-025-03070-w