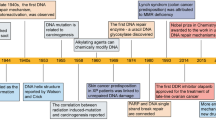
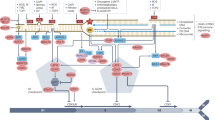
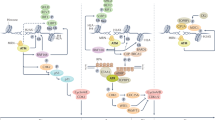
Abstract
Upon genotoxic stresses, cells employ various DNA damage responses (DDRs), including DNA damage repair or apoptosis, to safeguard genome integrity. However, the determinants among different DDRs choices are largely unknown. Here, we report angiopoietin-like protein 8 (ANGPTL8), a secreted regulator of lipid metabolism, localizes to the nucleus and acts as a dynamic switch that directs DDRs towards apoptosis rather than DNA repair after genotoxin exposure. ANGPTL8 deficiency alleviates DNA damage and apoptosis in cells exposed to genotoxins, as well as in the liver or kidney of mice injured by hepatic ischemia/reperfusion or cisplatin treatment. Mechanistically, ANGPTL8 physically interacts with Poly (ADP-ribose) polymerase 1 (PARP1), in a PARylation-independent manner, and reduces the fluidity of PARP1-DNA condensates, thereby enhancing the pro-apoptotic accumulation of PARP1 and PAR chains on DNA lesions. However, the transcription of ANGPTL8 is gradually decreased following genotoxin treatment, partly due to downregulation of CCAAT enhancer binding protein alpha (CEBPA), presumably to avoid further cytotoxicity. Together, we provide new insights by which genotoxic stress induced DDRs are channeled to suicidal apoptosis to safeguard genome integrity.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data relating to this study are included in the main text, figures, supplementary materials or available from the corresponding author upon reasonable request. Databases used in this study include Human Protein Atlas (https://www.proteinatlas.org), JASPAR (https://jaspar.genereg.net), hTFtarget (http:// bioinfo.life.hust.edu.cn/hTFtarget), Human TFBD 3.0 (http://bioinfo.life.hust.edu.cn/ HumanTFDB), as well as prediction tools for NLS (NLS Mapper (keio.ac.jp) and NES (LocNES NES prediction tool by Chook Lab (swmed.edu))
References
Tubbs A, Nussenzweig A. Endogenous DNA damage as a source of genomic instability in cancer. Cell. 2017;168:644–56.
O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547–60.
Ray Chaudhuri A, Nussenzweig A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat Rev Mol Cell Biol. 2017;18:610–21.
Pandey N, Black BE. Rapid detection and signaling of DNA damage by PARP-1. Trends Biochem Sci. 2021;46:744–57.
Kraus WL. PARPs and ADP-ribosylation: 60 years on. Genes Dev. 2020;34:251–3.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25:486–541.
Virag L. Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharm Rev. 2002;54:375–429.
Shirai H, Poetsch AR, Gunji A, Maeda D, Fujimori H, Fujihara H, et al. PARG dysfunction enhances DNA double strand break formation in S-phase after alkylation DNA damage and augments different cell death pathways. Cell Death Dis. 2013;4:e656.
Chen SH, Yu X. Targeting dePARylation selectively suppresses DNA repair-defective and PARP inhibitor-resistant malignancies. Sci Adv. 2019;5:eaav4340.
Koh DW, Lawler AM, Poitras MF, Sasaki M, Wattler S, Nehls MC, et al. Failure to degrade poly(ADP-ribose) causes increased sensitivity to cytotoxicity and early embryonic lethality. Proc Natl Acad Sci USA. 2004;101:17699–704.
Cortes U, Tong WM, Coyle DL, Meyer-Ficca ML, Meyer RG, Petrilli V, et al. Depletion of the 110-kilodalton isoform of poly(ADP-ribose) glycohydrolase increases sensitivity to genotoxic and endotoxic stress in mice. Mol Cell Biol. 2004;24:7163–78.
Min W, Cortes U, Herceg Z, Tong WM, Wang ZQ. Deletion of the nuclear isoform of poly(ADP-ribose) glycohydrolase (PARG) reveals its function in DNA repair, genomic stability and tumorigenesis. Carcinogenesis. 2010;31:2058–65.
Tufan AB, Lazarow K, Kolesnichenko M, Sporbert A, von Kries JP, Scheidereit C. TSG101 associates with PARP1 and is essential for PARylation and DNA damage-induced NF-kappaB activation. EMBO J. 2022;41:e110372.
Sethy C, Kundu CN. PARP inhibitor BMN-673 induced apoptosis by trapping PARP-1 and inhibiting base excision repair via modulation of pol-beta in chromatin of breast cancer cells. Toxicol Appl Pharm. 2022;436:115860.
Pommier Y, O’Connor MJ, de Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci Transl Med. 2016;8:362ps317.
Shao Z, Lee BJ, Rouleau-Turcotte E, Langelier MF, Lin X, Estes VM, et al. Clinical PARP inhibitors do not abrogate PARP1 exchange at DNA damage sites in vivo. Nucleic Acids Res. 2020;48:9694–709.
Luo X, Kraus WL. On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev. 2012;26:417–32.
Curtin NJ, Szabo C. Poly(ADP-ribose) polymerase inhibition: past, present and future. Nat Rev Drug Discov. 2020;19:711–36.
Mitrea DM, Mittasch M, Gomes BF, Klein IA, Murcko MA. Modulating biomolecular condensates: a novel approach to drug discovery. Nat Rev Drug Discov. 2022;21:841–62.
Chappidi N, Quail T, Doll S, Vogel LT, Aleksandrov R, Felekyan S, et al. PARP1-DNA co-condensation drives DNA repair site assembly to prevent disjunction of broken DNA ends. Cell. 2024;187:945–961 e918.
Altmeyer M, Neelsen KJ, Teloni F, Pozdnyakova I, Pellegrino S, Grofte M, et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat Commun. 2015;6:8088.
Patel A, Lee HO, Jawerth L, Maharana S, Jahnel M, Hein MY, et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell. 2015;162:1066–77.
Nosella ML, Kim TH, Huang SK, Harkness RW, Goncalves M, Pan A, et al. Poly(ADP-ribosyl)ation enhances nucleosome dynamics and organizes DNA damage repair components within biomolecular condensates. Mol Cell. 2024;84:429–46 e417.
Wei X, Zhou F, Zhang L. PARP1-DNA co-condensation: the driver of broken DNA repair. Signal Transduct Target Ther. 2024;9:135.
Shin Y, Brangwynne CP. Liquid phase condensation in cell physiology and disease. Science. 2017;357:eaaf4382.
Alberti S. Phase separation in biology. Curr Biol. 2017;27:R1097–R1102.
Abu-Farha M, Ghosh A, Al-Khairi I, Madiraju SRM, Abubaker J, Prentki M. The multi-faces of Angptl8 in health and disease: novel functions beyond lipoprotein lipase modulation. Prog Lipid Res. 2020;80:101067.
Yang J, Song QY, Niu SX, Chen HJ, Petersen RB, Zhang Y, et al. Emerging roles of angiopoietin-like proteins in inflammation: mechanisms and potential as pharmacological targets. J Cell Physiol. 2022;237:98–117.
Gusarova V, Banfi S, Alexa-Braun CA, Shihanian LM, Mintah IJ, Lee JS, et al. ANGPTL8 blockade with a monoclonal antibody promotes triglyceride clearance, energy expenditure, and weight loss in mice. Endocrinology. 2017;158:1252–9.
Vatner DF, Goedeke L, Camporez JG, Lyu K, Nasiri AR, Zhang D, et al. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia. 2018;61:1435–46.
Tseng YH, Ke PY, Liao CJ, Wu SM, Chi HC, Tsai CY, et al. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy. 2014;10:20–31.
Wang C, Tong Y, Wen Y, Cai J, Guo H, Huang L, et al. Hepatocellular carcinoma-associated protein TD26 interacts and enhances sterol regulatory element-binding protein 1 activity to promote tumor cell proliferation and growth. Hepatology. 2018;68:1833–50.
Zhang Y, Guo X, Yan W, Chen Y, Ke M, Cheng C, et al. ANGPTL8 negatively regulates NF-kappaB activation by facilitating selective autophagic degradation of IKKgamma. Nat Commun. 2017;8:2164.
Zhang Z, Wu H, Dai L, Yuan Y, Zhu Y, Ma Z, et al. ANGPTL8 enhances insulin sensitivity by directly activating insulin-mediated AKT phosphorylation. Gene. 2020;749:144707.
Sun X, Fu K, Hodgson A, Wier EM, Wen MG, Kamenyeva O, et al. Sam68 is required for DNA damage responses via regulating poly(ADP-ribosyl)ation. Plos Biol. 2016;14:e1002543.
Yang F, Chen JJ, Liu B, Gao GZ, Sebastian M, Jeter C, et al. SPINDOC binds PARP1 to facilitate PARylation. Nat Commun. 2021;12:6362.
Turinetto V, Giachino C. Multiple facets of histone variant H2AX: a DNA double-strand-break marker with several biological functions. Nucleic Acids Res. 2015;43:2489–98.
Abu-Qare AW, Abou-Donia MB. Biomarkers of apoptosis: release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2’-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J Toxicol Environ Health B Crit Rev. 2001;4:313–32.
Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–13.
Roos WP, Thomas AD, Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33.
Gunn A, Stark JM. I-SceI-based assays to examine distinct repair outcomes of mammalian chromosomal double strand breaks. Methods Mol Biol. 2012;920:379–91.
Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–99.
Rhine K, Odeh HM, Shorter J, Myong S. Regulation of biomolecular condensates by Poly(ADP-ribose). Chem Rev. 2023;123:9065–93.
Zhang L, Li DQ. MORC2 regulates DNA damage response through a PARP1-dependent pathway. Nucleic Acids Res. 2019;47:8502–20.
Lee YH, Lee SG, Lee CJ, Kim SH, Song YM, Yoon MR, et al. Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Sci Rep. 2016;6:24013.
Khandoga A, Enders G, Biberthaler P, Krombach F. Poly(ADP-ribose) polymerase triggers the microvascular mechanisms of hepatic ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2002;283:G553–560.
Konishi T, Lentsch AB. Hepatic ischemia/reperfusion: mechanisms of tissue injury, repair, and regeneration. Gene Expr. 2017;17:277–87.
Wang M, Zhang J, Zhang J, Sun K, Li Q, Kuang B, et al. Methyl eugenol attenuates liver ischemia reperfusion injury via activating PI3K/Akt signaling. Int Immunopharmacol. 2021;99:108023.
Matt S, Hofmann TG. The DNA damage-induced cell death response: a roadmap to kill cancer cells. Cell Mol Life Sci. 2016;73:2829–50.
Wang JYJ. Cell death response to DNA damage. Yale J Biol Med. 2019;92:771–9.
Kiraz Y, Adan A, Kartal Yandim M, Baran Y. Major apoptotic mechanisms and genes involved in apoptosis. Tumour Biol. 2016;37:8471–86.
Heymann F, Tacke F. Immunology in the liver-from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110.
Groelly FJ, Fawkes M, Dagg RA, Blackford AN, Tarsounas M. Targeting DNA damage response pathways in cancer. Nat Rev Cancer. 2023;23:78–94.
Lord CJ, Ashworth A. PARP inhibitors: synthetic lethality in the clinic. Science. 2017;355:1152–8.
Pilie PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res. 2019;25:3759–71.
Rose M, Burgess JT, O’Byrne K, Richard DJ, Bolderson E. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev Biol. 2020;8:564601.
Li H, Liu ZY, Wu N, Chen YC, Cheng Q, Wang J. PARP inhibitor resistance: the underlying mechanisms and clinical implications. Mol Cancer. 2020;19:107.
Zhang Y, Zhang ZT, Wan SY, Yang J, Wei YJ, Chen HJ, et al. ANGPTL3 negatively regulates IL-1beta-induced NF-kappaB activation by inhibiting the IL1R1-associated signaling complex assembly. J Mol Cell Biol. 2023;15:mjad053.
Huang Y, Xie Y, Yang D, Xiong M, Chen X, Wu D, et al. Histone demethylase UTX aggravates acetaminophen overdose induced hepatotoxicity through dual mechanisms. Pharm Res. 2022;175:106021.
Chen H, Liu C, Wang Q, Xiong M, Zeng X, Yang D, et al. Renal UTX-PHGDH-serine axis regulates metabolic disorders in the kidney and liver. Nat Commun. 2022;13:3835.
Wang J, Xiong MR, Fan Y, Liu CY, Wang Q, Yang D, et al. Mecp2 protects kidney from ischemia-reperfusion injury through transcriptional repressing IL-6/STAT3 signaling. Theranostics. 2022;12:3896–910.
Cui N, Liu C, Tang X, Song L, Xiao Z, Wang C, et al. ISG15 accelerates acute kidney injury and the subsequent AKI-to-CKD transition by promoting TGFbetaR1 ISGylation. Theranostics 2024;14:4536–53.
Yang C, Xu H, Yang D, Xie Y, Xiong M, Fan Y, et al. A renal YY1-KIM1-DR5 axis regulates the progression of acute kidney injury. Nat Commun. 2023;14:4261.
Chen H, Wang L, Wang WJ, Cheng C, Zhang Y, Zhou Y, et al. ELABELA and an ELABELA fragment protect against AKI. J Am Soc Nephrol. 2017;28:2695–708.
Chen Y, Shi J, Wang X, Zhou L, Wang Q, Xie Y, et al. An antioxidant feedforward cycle coordinated by linker histone variant H1.2 and NRF2 that drives nonsmall cell lung cancer progression. Proc Natl Acad Sci USA. 2023;120:e2306288120.
Xiong M, Chen H, Fan Y, Jin M, Yang D, Chen Y, et al. Tubular Elabela-APJ axis attenuates ischemia-reperfusion induced acute kidney injury and the following AKI-CKD transition by protecting renal microcirculation. Theranostics. 2023;13:3387–401.
Li X, Yu L, Liu X, Shi T, Zhang Y, Xiao Y, et al. Beta-synuclein regulates the phase transitions and amyloid conversion of alpha-synuclein. Nat Commun. 2024;15:8748.
Langelier MF, Planck JL, Servent KM, Pascal JM. Purification of human PARP-1 and PARP-1 domains from Escherichia coli for structural and biochemical analysis. Methods Mol Biol. 2011;780:209–26.
Liu C, Wang J, Wei Y, Zhang W, Geng M, Yuan Y, et al. Fat-specific knockout of Mecp2 upregulates slpi to reduce obesity by enhancing browning. Diabetes. 2020;69:35–47.
Yang D, Fan Y, Xiong M, Chen Y, Zhou Y, Liu X, et al. Loss of renal tubular G9a benefits acute kidney injury by lowering focal lipid accumulation via CES1. EMBO Rep. 2023;24:e56128.
Lou J, Chen H, Han J, He H, Huen MSY, Feng XH, et al. AUNIP/C1orf135 directs DNA double-strand breaks towards the homologous recombination repair pathway. Nat Commun. 2017;8:985.
Acknowledgements
We thank Drs. Xue-feng Chen and Qiang Chen from Wuhan University for reagents, technical help and stimulating discussions. We appreciate the technical support provided by the Huazhong University of Science & Technology Analytical & Testing center, as well as the Medical Sub-center of Analytical & Testing center of the Huazhong University of Science & Technology.
Funding
This work was supported by the National Key R&D Program of China (2023YFC2507900, 2022YFA0806100), the Natural Science Foundation of China (82273838 & 31971066), and the Natural Science Foundation of Hubei Province (2021CFA004), and also by the Tongji-Rong Cheng Biomedical Center.
Author information
Authors and Affiliations
Contributions
JY, Y Zhang, and KH designed the research and analyzed the data; JY, Y Zhang., SW, QS, Y Xie, JW, Y Zhou, and ZZ performed the experiments; Y Xiao, X Li, HC, X Liu, LX, HY, DH, LZ, and Y-H Zhang provide technical assistance of in vitro experiments or animal models; JY, RBP, Y Zhang and KH wrote the manuscript. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
No patient data were utilized in this study. Animal ethics are listed in the ‘Material and Methods’ section.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, J., Wan, Sy., Song, Qy. et al. Angiopoietin-like protein 8 directs DNA damage responses towards apoptosis by stabilizing PARP1-DNA condensates. Cell Death Differ 32, 672–688 (2025). https://doi.org/10.1038/s41418-024-01422-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-024-01422-2