Abstract
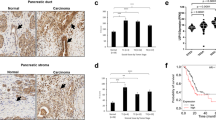
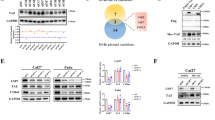
The ubiquitin-specific protease (USP) family is the largest and most diverse deubiquitinase (DUBs) family and plays a significant role in maintaining cell homeostasis. Dysregulation of USPs has been associated with carcinogenesis of various tumors. We identified that USP19 was downregulated in pancreatic tumor tissues and forced expression of USP19 diminished tumorigenicity of pancreatic cancer. Mechanistically, USP19 directly interacts with and stabilized NEK9 via inhibiting K48-specific polyubiquitination process on NEK9 protein at K525 site through its USP ___domain. Moreover, NEK9 phosphorylates the regulatory associated protein of mTOR (Raptor) at Ser792 and links USP19 to the inhibition of mTORC1 signaling pathway, which further leads to autophagic cell death of pancreatic cancer cells. Inhibition of autophagy by Atg5 knockdown or lysosome inhibitor bafilomycin A1 abolished the decreased malignant phenotype of USP19- and NEK9-overexpressing cancer cells. Importantly, USP19 expression exhibits a positive correlation with NEK9 expression in clinical samples, and low USP19 or NEK9 expression is associated with a worse prognosis. This study revealed that USP19-mediated NEK9 deubiquitylation is a regulatory mechanism for mTORC1 inhibition and provides a therapeutic target for diseases involving mTORC1 dysregulation.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All datasets are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Kitamura H. Ubiquitin-specific proteases (USPs) and metabolic disorders. Int J Mol Sci. 2023;24:3219.
Bonacci T, Emanuele MJ. Dissenting degradation: deubiquitinases in cell cycle and cancer. Semin Cancer Biol. 2020;67:145–58.
Jin S, Tian S, Chen Y, Zhang C, Xie W, Xia X, et al. USP19 modulates autophagy and antiviral immune responses by deubiquitinating Beclin-1. EMBO J. 2016;35:866–80.
Zhu Y, Gu L, Lin X, Zhou X, Lu B, Liu C, et al. USP19 exacerbates lipogenesis and colorectal carcinogenesis by stabilizing ME1. Cell Rep. 2021;37:110174.
Zhang X, Chen X, Qian F, Zhu Y, He G, Yang J, et al. Deubiquitinase USP19 modulates apoptotic calcium release and endoplasmic reticulum stress by deubiquitinating BAG6 in triple negative breast cancer. Clin Transl Med. 2023;13:e1398.
Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17:528–42.
Yamamoto Y, Chino H, Tsukamoto S, Ode KL, Ueda HR, Mizushima N. NEK9 regulates primary cilia formation by acting as a selective autophagy adaptor for MYH9/myosin IIA. Nat Commun. 2021;12:3292.
Behrends C, Sowa ME, Gygi SP, Harper JW. Network organization of the human autophagy system. Nature. 2010;466:68–76.
Shrestha BK, Skytte Rasmussen M, Abudu YP, Bruun JA, Larsen KB, Alemu EA, et al. NIMA-related kinase 9-mediated phosphorylation of the microtubule-associated LC3B protein at Thr-50 suppresses selective autophagy of p62/sequestosome 1. J Biol Chem. 2020;295:1240–60.
Panwar V, Singh A, Bhatt M, Tonk RK, Azizov S, Raza AS, et al. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. 2023;8:375.
Nakatogawa H. Mechanisms governing autophagosome biogenesis. Nat Rev Mol Cell Biol. 2020;21:439–58.
Ding WX, Ma X, Kim S, Wang S, Ni HM. Recent insights about autophagy in pancreatitis. eGastroenterology. 2024;2:e100057.
Hernandez GA, Perera RM. Autophagy in cancer cell remodeling and quality control. Mol Cell. 2022;82:1514–27.
Yang Y, Zhang Y, Gao J, Xu W, Xu Z, Li Z, et al. Pyrethrum extract induces oxidative DNA damage and AMPK/mTOR-mediated autophagy in SH-SY5Y cells. Sci Total Environ. 2020;740:139925.
Liu W, Zhao Y, Wang G, Feng S, Ge X, Ye W, et al. TRIM22 inhibits osteosarcoma progression through destabilizing NRF2 and thus activation of ROS/AMPK/mTOR/autophagy signaling. Redox Biol. 2022;53:102344.
Jia S, Xu X, Zhou S, Chen Y, Ding G, Cao L. Fisetin induces autophagy in pancreatic cancer cells via endoplasmic reticulum stress- and mitochondrial stress-dependent pathways. Cell Death Dis. 2019;10:142.
Szwed A, Kim E, Jacinto E. Regulation and metabolic functions of mTORC1 and mTORC2. Physiol Rev. 2021;101:1371–426.
Wang G, Gao H, Dai S, Li M, Gao Y, Yin L, et al. Metformin inhibits neutrophil extracellular traps-promoted pancreatic carcinogenesis in obese mice. Cancer Lett. 2023;562:216155.
Wang G, Yin L, Peng Y, Gao Y, Gao H, Zhang J, et al. Insulin promotes invasion and migration of KRAS(G12D) mutant HPNE cells by upregulating MMP-2 gelatinolytic activity via ERK- and PI3K-dependent signalling. Cell Prolif. 2019;52:e12575.
Bononi G, Masoni S, Di Bussolo V, Tuccinardi T, Granchi C, Minutolo F. Historical perspective of tumor glycolysis: a century with Otto Warburg. Semin Cancer Biol. 2022;86:325–33.
Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684.
Weng ML, Chen WK, Chen XY, Lu H, Sun ZR, Yu Q, et al. Fasting inhibits aerobic glycolysis and proliferation in colorectal cancer via the Fdft1-mediated AKT/mTOR/HIF1alpha pathway suppression. Nat Commun. 2020;11:1869.
Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–83.
Perl A. mTOR activation is a biomarker and a central pathway to autoimmune disorders, cancer, obesity, and aging. Ann N Y Acad Sci. 2015;1346:33–44.
Fan H, Wu Y, Yu S, Li X, Wang A, Wang S, et al. Critical role of mTOR in regulating aerobic glycolysis in carcinogenesis (Review). Int J Oncol. 2021;58:9–19.
Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–26.
Chandrasekaran AP, Tyagi A, Poondla N, Sarodaya N, Karapurkar JK, Kaushal K, et al. Dual role of deubiquitinating enzyme USP19 regulates mitotic progression and tumorigenesis by stabilizing survivin. Mol Ther. 2022;30:3414–29.
Tyagi A, Karapurkar JK, Colaco JC, Sarodaya N, Antao AM, Kaushal K, et al. USP19 negatively regulates p53 and promotes cervical cancer progression. Mol Biotechnol. 2023;66:2032–45.
Hu W, Su Y, Fei X, Wang X, Zhang G, Su C, et al. Ubiquitin specific peptidase 19 is a prognostic biomarker and affect the proliferation and migration of clear cell renal cell carcinoma. Oncol Rep. 2020;43:1964–74.
Bertran MT, Sdelci S, Regue L, Avruch J, Caelles C, Roig J. Nek9 is a Plk1-activated kinase that controls early centrosome separation through Nek6/7 and Eg5. EMBO J. 2011;30:2634–47.
Lu G, Du R, Dong J, Sun Y, Zhou F, Feng F, et al. Cancer associated fibroblast derived SLIT2 drives gastric cancer cell metastasis by activating NEK9. Cell Death Dis. 2023;14:421.
Lu G, Tian S, Sun Y, Dong J, Wang N, Zeng J, et al. NEK9, a novel effector of IL-6/STAT3, regulates metastasis of gastric cancer by targeting ARHGEF2 phosphorylation. Theranostics. 2021;11:2460–74.
Gao WL, Niu L, Chen WL, Zhang YQ, Huang WH. Integrative analysis of the expression levels and prognostic Values for NEK family members in breast cancer. Front Genet. 2022;13:798170.
Nie H, Huang PQ, Jiang SH, Yang Q, Hu LP, Yang XM, et al. The short isoform of PRLR suppresses the pentose phosphate pathway and nucleotide synthesis through the NEK9-Hippo axis in pancreatic cancer. Theranostics. 2021;11:3898–915.
Liu Y, Wang X, Zhu W, Sui Z, Wei X, Zhang Y, et al. TRPML1-induced autophagy inhibition triggers mitochondrial mediated apoptosis. Cancer Lett. 2022;541:215752.
Sang J, Gan L, Zou MF, Lin ZJ, Fan RZ, Huang JL, et al. Jolkinolide B sensitizes bladder cancer to mTOR inhibitors via dual inhibition of Akt signaling and autophagy. Cancer Lett. 2022;526:352–62.
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye WC, et al. Autophagy and multidrug resistance in cancer. Chin J Cancer. 2017;36:52.
Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605–16.
Cui J, Jin S, Wang RF. The BECN1-USP19 axis plays a role in the crosstalk between autophagy and antiviral immune responses. Autophagy. 2016;12:1210–1.
Zhao X, Di Q, Yu J, Quan J, Xiao Y, Zhu H, et al. USP19 (ubiquitin specific peptidase 19) promotes TBK1 (TANK-binding kinase 1) degradation via chaperone-mediated autophagy. Autophagy. 2022;18:891–908.
Funding
This work was supported by the grant from the Natural Science Foundation of Jiangsu Province (BK20221416), Clinical Science and Technology Climbing program - “Spark” basic research project (ZJ202124), Young Scholars Fostering Fund of the First Affiliated Hospital of Nanjing Medical University (PY202404) and Innovation Capability Development Project of Jiangsu Province (No. BM2015004). All samples used in current study were obtained from Pancreas Biobank, The First Affiliated Hospital with Nanjing Medical University, which is a part of Jiangsu Biobank of Clinical Resource.
Author information
Authors and Affiliations
Contributions
Conception and design: GFW; data acquisition, analysis, and interpretation: GFW, SND, JC, KZ; investigation: GFW, SND, JC, KZ, JFZ, FYL, HJW; methodology: KZ, ZPL, CYH, LDY; acquisition of patient specimens: JFZ, KXX, FYL, HJW, YG; supervision: ZPL, YM, KRJ; resources: ZPL, YM, JC; and article drafting and revising: GFW, SND. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement and consent to participate
All participants provided informed consent. All human tissue research in this study had the approval of ethics committees of the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). All animal experiments were performed in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the Nanjing Medical University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, G., Dai, S., Chen, J. et al. USP19 potentiates autophagic cell death via inhibiting mTOR pathway through deubiquitinating NEK9 in pancreatic cancer. Cell Death Differ 32, 702–713 (2025). https://doi.org/10.1038/s41418-024-01426-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-024-01426-y