Abstract
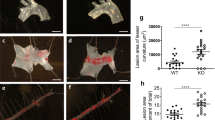
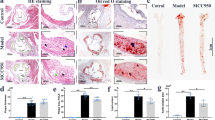
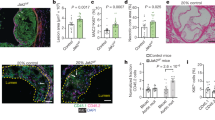
Inflammation plays a crucial role in the progression of atherosclerosis. Junctional adhesion molecule-like protein (JAML), a type-I transmembrane glycoprotein, activates downstream signaling pathways. However, the precise role of macrophage-derived JAML in inflammation and atherosclerosis remains unclear. This study aimed to generate mice with macrophage-specific deletion or overexpression of JAML, with the focus of assessing its impact on macrophage function and elucidating its regulatory mechanism in atherosclerosis. High-throughput data screening was employed to investigate JAML expression in atherosclerosis, and macrophage-specific JAML-knockout and transgenic mice models were utilized to examine the effects of JAML on atherosclerosis. Furthermore, the role of JAML was assessed using Oil Red O staining, RNA-sequencing analysis, and co-immunoprecipitation techniques. Increased JAML expression was observed in macrophages from both mice and patients with atherosclerosis. Macrophage-specific JAML deletion attenuated atherosclerosis and inflammation, whereas macrophage-specific JAML overexpression exacerbated these conditions. Mechanistically, JAML deletion inhibited inflammation by decreasing nuclear translocation of pyruvate kinase M2 (PKM2) and PKM2/p65 complex formation, which consequently suppressed the nuclear factor kappa B (NF-κB) pathway and NLRP3 inflammasome activation. Taken together, these findings demonstrate that macrophage-expressed JAML facilitates the progression of atherosclerosis by activating the NF-κB pathway and NLRP3 inflammasome through nuclear migration and phosphorylation of PKM2. Notably, our study revealed a novel mechanism for the regulation of NLRP3 inflammasome activation in atherosclerosis. Therefore, targeting JAML may be an effective treatment strategy for atherosclerosis, a condition characterized by chronic inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Original data of RNA-sequencing are available from the NCBI Gene Expression Omnibus (GEO) database under accession number GSE287332.
References
Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22. https://doi.org/10.1016/S0140-6736(20)30925-9
Riksen NP, Bekkering S, Mulder WJM, Netea MG. Trained immunity in atherosclerotic cardiovascular disease. Nat Rev Cardiol. 2023;20:799–811. https://doi.org/10.1038/s41569-023-00894-y
Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–65. https://doi.org/10.1146/annurev.immunol.021908.132715
Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. https://doi.org/10.1038/nature08938
Verdino P, Witherden DA, Havran WL, Wilson IA. The molecular interaction of CAR and JAML recruits the central cell signal transducer PI3K. Science. 2010;329:1210–4. https://doi.org/10.1126/science.1187996
Witherden DA, Verdino P, Rieder SE, Garijo O, Mills RE, Teyton L, et al. The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science. 2010;329:1205–10. https://doi.org/10.1126/science.1192698
Wu Q, Li R, Wang QX, Zhang MY, Liu TT, Qu YQ. Junctional adhesion molecule-like protein promotes tumor progression via the Wnt/beta-catenin signaling pathway in lung adenocarcinoma. J Transl Med. 2022;20:260. https://doi.org/10.1186/s12967-022-03457-w
Mraz V, Lohmann RKD, Menzel M, Hawkes A, Vaher H, Funch AB, et al. The junctional adhesion molecule-like protein (JAML) is important for the inflammatory response during contact hypersensitivity. Contact Dermatitis. 2023;89:323–34. https://doi.org/10.1111/cod.14409
Moog-Lutz C, Cave- RiantF, Guibal FC, Breau MA, Di Gioia Y, Couraud PO, et al. JAML, a novel protein with characteristics of a junctional adhesion molecule, is induced during differentiation of myeloid leukemia cells. Blood. 2003;102:3371–8. https://doi.org/10.1182/blood-2002-11-3462
Elo H, Kuure M, Pelttari E. Correlation of the antimicrobial activity of salicylaldehydes with broadening of the NMR signal of the hydroxyl proton. Possible involvement of proton exchange processes in the antimicrobial activity. Eur J Med Chem. 2015;92:750–3. https://doi.org/10.1016/j.ejmech.2015.01.041
Alvarez JI, Kebir H, Cheslow L, Charabati M, Chabarati M, Larochelle C, et al. JAML mediates monocyte and CD8 T cell migration across the brain endothelium. Ann Clin Transl Neurol. 2015;2:1032–7. https://doi.org/10.1002/acn3.255
Fu Y, Sun Y, Wang M, Hou Y, Huang W, Zhou D, et al. Elevation of JAML promotes diabetic kidney disease by modulating podocyte lipid metabolism. Cell Metab. 2020;32:1052–62.e8. https://doi.org/10.1016/j.cmet.2020.10.019
Weber DA, Sumagin R, McCall IC, Leoni G, Neumann PA, Andargachew R, et al. Neutrophil-derived JAML inhibits repair of intestinal epithelial injury during acute inflammation. Mucosal Immunol. 2014;7:1221–32. https://doi.org/10.1038/mi.2014.12
Eschweiler S, Wang A, Ramirez-Suastegui C, von Witzleben A, Li Y, Chee SJ, et al. JAML immunotherapy targets recently activated tumor-infiltrating CD8(+) T cells. Cell Rep. 2023;42:112040. https://doi.org/10.1016/j.celrep.2023.112040
Fang Y, Yang J, Zu G, Cong C, Liu S, Xue F, et al. Junctional adhesion molecule-like protein promotes tumor progression and metastasis via p38 signaling pathway in gastric cancer. Front Oncol. 2021;11:565676 https://doi.org/10.3389/fonc.2021.565676
Sun Y, Guan J, Hou Y, Xue F, Huang W, Zhang W, et al. Silencing of junctional adhesion molecule-like protein attenuates atherogenesis and enhances plaque stability in ApoE(-/-) mice. Clin Sci. 2019;133:1215–28. https://doi.org/10.1042/CS20180561
Zeng W, Wu D, Sun Y, Suo Y, Yu Q, Zeng M, et al. The selective NLRP3 inhibitor MCC950 hinders atherosclerosis development by attenuating inflammation and pyroptosis in macrophages. Sci Rep. 2021;11:19305. https://doi.org/10.1038/s41598-021-98437-3
Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55. https://doi.org/10.1038/nm.3806
Mashayekhi V, Schomisch A, Rasheed S, Aparicio-Puerta E, Risch T, Yildiz D, et al. The RNA binding protein IGF2BP2/IMP2 alters the cargo of cancer cell-derived extracellular vesicles supporting tumor-associated macrophages. Cell Commun Signal. 2024;22:344. https://doi.org/10.1186/s12964-024-01701-y
Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol. 2012;188:5752–65. https://doi.org/10.4049/jimmunol.1103426
Niyonzima N, Bakke SS, Gregersen I, Holm S, Sandanger O, Orrem HL, et al. Cholesterol crystals use complement to increase NLRP3 signaling pathways in coronary and carotid atherosclerosis. EBioMedicine. 2020;60:102985. https://doi.org/10.1016/j.ebiom.2020.102985
Samstad EO, Niyonzima N, Nymo S, Aune MH, Ryan L, Bakke SS, et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J Immunol. 2014;192:2837–45. https://doi.org/10.4049/jimmunol.1302484
Cole JE, Park I, Ahern DJ, Kassiteridi C, Danso Abeam D, Goddard ME, et al. Immune cell census in murine atherosclerosis: cytometry by time of flight illuminates vascular myeloid cell diversity. Cardiovasc Res. 2018;114:1360–71. https://doi.org/10.1093/cvr/cvy109
Nozaki K, Li L, Miao EA. Innate sensors trigger regulated cell death to combat intracellular infection. Annu Rev Immunol. 2022;40:469–98. https://doi.org/10.1146/annurev-immunol-101320-011235
Fu J, Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Annu Rev Immunol. 2023;41:301–16. https://doi.org/10.1146/annurev-immunol-081022-021207
Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20:3328. https://doi.org/10.3390/ijms20133328
Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–61. https://doi.org/10.1038/ni.1836
Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat Immunol. 2013;14:812–20. https://doi.org/10.1038/ni.2639
Li H, Guan Y, Liang B, Ding P, Hou X, Wei W, et al. Therapeutic potential of MCC950, a specific inhibitor of NLRP3 inflammasome. Eur J Pharmacol. 2022;928:175091. https://doi.org/10.1016/j.ejphar.2022.175091
Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122:1722–40. https://doi.org/10.1161/CIRCRESAHA.118.311362
Zhang Z, Deng X, Liu Y, Liu Y, Sun L, Chen F. PKM2, function and expression and regulation. Cell Biosci. 2019;9:52 https://doi.org/10.1186/s13578-019-0317-8
Doddapattar P, Dev R, Ghatge M, Patel RB, Jain M, Dhanesha N, et al. Myeloid cell PKM2 deletion enhances efferocytosis and reduces atherosclerosis. Circ Res. 2022;130:1289–305. https://doi.org/10.1161/CIRCRESAHA.121.320704
Rao J, Wang H, Ni M, Wang Z, Wang Z, Wei S, et al. FSTL1 promotes liver fibrosis by reprogramming macrophage function through modulating the intracellular function of PKM2. Gut. 2022;71:2539–50. https://doi.org/10.1136/gutjnl-2021-325150
Wang P, Sun C, Zhu T, Xu Y. Structural insight into mechanisms for dynamic regulation of PKM2. Protein Cell. 2015;6:275–87. https://doi.org/10.1007/s13238-015-0132-x
Han D, Wei W, Chen X, Zhang Y, Wang Y, Zhang J, et al. NF-kappaB/RelA-PKM2 mediates inhibition of glycolysis by fenofibrate in glioblastoma cells. Oncotarget. 2015;6:26119–28. https://doi.org/10.18632/oncotarget.4444
Xu Q, Liu LZ, Yin Y, He J, Li Q, Qian X, et al. Regulatory circuit of PKM2/NF-kappaB/miR-148a/152-modulated tumor angiogenesis and cancer progression. Oncogene. 2015;34:5482–93. https://doi.org/10.1038/onc.2015.6
Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. https://doi.org/10.1126/scisignal.2000431
Fan L, Liu J, Zhang Y, Zhang C, Shi B, Hu X, et al. High-dimensional single-cell analysis delineates peripheral immune signature of coronary atherosclerosis in human blood. Theranostics. 2022;12:6809–25. https://doi.org/10.7150/thno.73336
Huang W, Wang BO, Hou YF, Fu Y, Cui SJ, Zhu JH, et al. JAML promotes acute kidney injury mainly through a macrophage-dependent mechanism. JCI Insight. 2022;7:e158571. https://doi.org/10.1172/jci.insight.158571
Christ A, Gunther P, Lauterbach MAR, Duewell P, Biswas D, Pelka K, et al. Western diet triggers NLRP3-dependent innate immune reprogramming. Cell. 2018;172:162–175.e14. https://doi.org/10.1016/j.cell.2017.12.013
Qiao L, Ma J, Zhang Z, Sui W, Zhai C, Xu D, et al. Deficient chaperone-mediated autophagy promotes inflammation and atherosclerosis. Circ Res. 2021;129:1141–57. https://doi.org/10.1161/CIRCRESAHA.121.318908
Li X, Tian BM, Deng DK, Liu F, Zhou H, Kong DQ, et al. LncRNA GACAT2 binds with protein PKM1/2 to regulate cell mitochondrial function and cementogenesis in an inflammatory environment. Bone Res. 2022;10:29. https://doi.org/10.1038/s41413-022-00197-x
Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun. 2016;7:13280. https://doi.org/10.1038/ncomms13280
Kim H, Takegahara N, Choi Y. IgSF11-mediated phosphorylation of pyruvate kinase M2 regulates osteoclast differentiation and prevents pathological bone loss. Bone Res. 2023;11:17. https://doi.org/10.1038/s41413-023-00251-2
Gai X, Liu F, Wu Y, Zhang B, Tang B, Shang K, et al. Overexpressed PKM2 promotes macrophage phagocytosis and atherosclerosis. Animal Model Exp Med. 2023;6:92–102. https://doi.org/10.1002/ame2.12266
Lu S, Deng J, Liu H, Liu B, Yang J, Miao Y, et al. PKM2-dependent metabolic reprogramming in CD4(+) T cells is crucial for hyperhomocysteinemia-accelerated atherosclerosis. J Mol Med. 2018;96:585–600. https://doi.org/10.1007/s00109-018-1645-6
Funding
This work was supported by grants of the National Natural Science Foundation of China (Nos. 81100207), the Natural Science Foundation of Shandong Province (ZR2024ZD23 and ZR2024ZD09) and the Taishan Scholar Project of Shandong Province of China (No. tstp20240852).
Author information
Authors and Affiliations
Contributions
JY, WZ and CZ conceived, designed the study, and revised the pre-final manuscript and final approval of the version. HC, LX and HL contributed to data acquisition, analysis and writing of the manuscript. CC, FX, ZW, LL and LQ participated in data curation and statistical analysis. All authors have read the manuscript and provided comments and suggestions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All animal experimental protocols adhered to the Management Rules of the Chinese Ministry of Health and were approved by the appropriate Ethics Committee (DWLL-2023-037). All human experimental protocols were approved by the Ethics Committee of Shandong University Qilu Hospital. Written informed consent was obtained from all the participants, and the study was conducted in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, H., Xie, L., Lu, H. et al. Macrophage junctional adhesion molecule-like (JAML) protein promotes NLRP3 inflammasome activation in the development of atherosclerosis. Cell Death Differ (2025). https://doi.org/10.1038/s41418-025-01489-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41418-025-01489-5