Summary
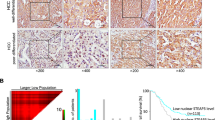
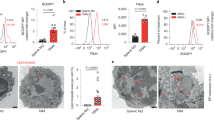
TSPAN family of proteins are generally considered to assemble as multimeric complexes on the plasma membrane. Our previous work uncovered that TSPAN8 can translocate into the nucleus as a membrane-free form, a process that requires TSPAN8 palmitoylation and association with cholesterol to promote its extraction from the plasma membrane and subsequent binding with 14-3-3θ and importin-β. However, what upstream signal(s) regulate(s) the nuclear translocation of TSPAN8, the potential function of TSPAN8 in the nucleus, and the underlying molecular mechanisms all remain unclear. Here, we demonstrate that, epidermal growth factor receptor (EGFR) signaling induces TSPAN8 nuclear translocation by activating the kinase AKT, which in turn directly phosphorylates TSPAN8 at Ser129, an event essential for its binding with 14-3-3θ and importin ß1. In the nucleus, phosphorylated TSPAN8 interacts with STAT3 to enhance its chromatin occupancy and therefore regulates transcription of downstream cancer-promoting genes, such as MYC, BCL2, MMP9, etc. The EGFR–AKT–TSPAN8–STAT3 axis was found to be hyperactivated in multiple human cancers, and associated with aggressive phenotype and dismal prognosis. We further developed a humanized monoclonal antibody hT8Ab4 that specifically recognizes the large extracellular loop of TSPAN8 (TSPAN8-LEL), thus being able to block the extraction of TSPAN8 from the plasma membrane and consequently its nuclear localization. Importantly, both in vitro and in vivo studies demonstrated an antitumor effect of hT8Ab4. Collectively, we discovered an unconventional function of TSPAN8 and dissected the underlying molecular mechanisms, which not only showcase a new layer of biological complexity of traditional membrane proteins, but also shed light on TSPAN8 as a novel therapeutic target for refractory cancers.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Zeng, A. et al. Prospectively isolated tetraspanin(+) neoblasts are adult pluripotent stem cells underlying planaria regeneration. Cell 173, 1593–1608.e20 (2018).
Zimmerman, B. et al. Crystal structure of a full-length human tetraspanin reveals a cholesterol-binding pocket. Cell 167, 1041–1051.e11 (2016).
Yang, Y. et al. Open conformation of tetraspanins shapes interaction partner networks on cell membranes. EMBO J. 39, e105246 (2020).
Huang, Y. et al. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat. Cell Biol. 21, 991–1002 (2019).
Yanez-Mo, M., Barreiro, O., Gordon-Alonso, M., Sala-Valdes, M. & Sanchez-Madrid, F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 19, 434–446 (2009).
Liu, Z. et al. Tetraspanins TSP-12 and TSP-14 function redundantly to regulate the trafficking of the type II BMP receptor in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 117, 2968–2977 (2020).
Nankivell, P. et al. Tetraspanins CD9 and CD151, epidermal growth factor receptor and cyclooxygenase-2 expression predict malignant progression in oral epithelial dysplasia. Br. J. Cancer 109, 2864–2874 (2013).
Liu, Z. et al. Promotion of bone morphogenetic protein signaling by tetraspanins and glycosphingolipids. PLoS Genet. 11, e1005221 (2015).
Levy, S. & Shoham, T. The tetraspanin web modulates immune-signalling complexes. Nat. Rev. Immunol. 5, 136–148 (2005).
Hemler, M. E. Targeting of tetraspanin proteins-potential benefits and strategies. Nat. Rev. Drug Discov. 7, 747–758 (2008).
Hemler, M. E. Tetraspanin proteins promote multiple cancer stages. Nat. Rev. Cancer 14, 49–60 (2014).
Wu, D. et al. Pairing of integrins with ECM proteins determines migrasome formation. Cell Res. 27, 1397–1400 (2017).
Zhang, S., Kodys, K., Babcock, G. J. & Szabo, G. CD81/CD9 tetraspanins aid plasmacytoid dendritic cells in recognition of hepatitis C virus-infected cells and induction of interferon-alpha. Hepatology 58, 940–949 (2013).
Tomlinson, M. G. Eye-opening potential for tetraspanin Tspan12 as a therapeutic target for diseases of the retinal vasculature. Circulation 136, 196–199 (2017).
Zoller, M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 9, 40–55 (2009).
McAllister, S. S. & Weinberg, R. A. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat. Cell Biol. 16, 717–727 (2014).
Greco, C. et al. E-cadherin/p120-catenin and tetraspanin Co-029 cooperate for cell motility control in human colon carcinoma. Cancer Res. 70, 7674–7683 (2010).
Berthier-Vergnes, O. et al. Gene expression profiles of human melanoma cells with different invasive potential reveal TSPAN8 as a novel mediator of invasion. Br. J. Cancer 104, 155–165 (2011).
Zhang, H. S., Liu, H. Y., Zhou, Z., Sun, H. L. & Liu, M. Y. TSPAN8 promotes colorectal cancer cell growth and migration in LSD1-dependent manner. Life Sci. 241, 117114 (2020).
Cheng, I. et al. Type 2 diabetes risk variants and colorectal cancer risk: the Multiethnic Cohort and PAGE studies. Gut 60, 1703–1711 (2011).
Schafer, D. et al. Identification of CD318, TSPAN8 and CD66c as target candidates for CAR T cell based immunotherapy of pancreatic adenocarcinoma. Nat. Commun. 12, 1453 (2021).
Liu, Y. et al. Extracellular vesicle tetraspanin-8 level predicts distant metastasis in non-small cell lung cancer after concurrent chemoradiation. Sci. Adv. 6, eaaz6162 (2020).
Zhu, R. et al. TSPAN8 promotes cancer cell stemness via activation of sonic Hedgehog signaling. Nat. Commun. 10, 2863 (2019).
Fu, N. Y. et al. Identification of quiescent and spatially restricted mammary stem cells that are hormone responsive. Nat. Cell Biol. 19, 164–176 (2017).
Wang, H., Rana, S., Giese, N., Buchler, M. W. & Zoller, M. Tspan8, CD44v6 and alpha6beta4 are biomarkers of migrating pancreatic cancer-initiating cells. Int. J. Cancer 133, 416–426 (2013).
Li, J. et al. SOX9 is a critical regulator of TSPAN8-mediated metastasis in pancreatic cancer. Oncogene 40, 4884–4893 (2021).
Huang, Y. et al. Nuclear translocation of the 4-pass transmembrane protein Tspan8. Cell Res. 31, 1218–1221 (2021).
Schmidt, F. et al. Tetraspanin 8 is an interactor of the metalloprotease meprin beta within tetraspanin-enriched microdomains. Biol. Chem. 397, 857–869 (2016).
Yang, Y. C. et al. Circulating proteoglycan endocan mediates EGFR-driven progression of non-small cell lung cancer. Cancer Res. 80, 3292–3304 (2020).
Pan, M. et al. EpCAM ectodomain EpEX is a ligand of EGFR that counteracts EGF-mediated epithelial-mesenchymal transition through modulation of phospho-ERK1/2 in head and neck cancers. PLoS Biol. 16, e2006624 (2018).
Claperon, A. et al. Hepatic myofibroblasts promote the progression of human cholangiocarcinoma through activation of epidermal growth factor receptor. Hepatology 58, 2001–2011 (2013).
Ponsioen, B. et al. Quantifying single-cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling. Nat. Cell Biol. 23, 377–390 (2021).
Gao, M. et al. EGFR activates a TAZ-driven oncogenic program in glioblastoma. Cancer Res. 81, 3580–3592 (2021).
Luo, H. et al. ALCAM-EGFR interaction regulates myelomagenesis. Blood Adv. 5, 5269–5282 (2021).
Sigismund, S. et al. Clathrin-independent endocytosis of ubiquitinated cargos. Proc. Natl. Acad. Sci. USA 102, 2760–2765 (2005).
Akdag, M. et al. Proximal biotinylation-based combinatory approach for isolating integral plasma membrane proteins. J. Proteome Res. 19, 3583–3592 (2020).
Wei, W. et al. Cell type-selective secretome profiling in vivo. Nat. Chem. Biol. 17, 326–334 (2021).
Bogdan, S. & Klambt, C. Epidermal growth factor receptor signaling. Curr. Biol. 11, R292–R295 (2001).
Roux, K. J., Kim, D. I., Raida, M. & Burke, B. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. J. Cell. Biol. 196, 801–810 (2012).
Pekarsky, Y. et al. Tcl1 enhances Akt kinase activity and mediates its nuclear translocation. Proc. Natl. Acad. Sci. USA 97, 3028–3033 (2000).
Manning, B. D. & Cantley, L. C. AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 (2007).
Rouillard, A. D. et al. The harmonizome: a collection of processed datasets gathered to serve and mine knowledge about genes and proteins. Database 2016, baw100 (2016).
Iwamaru, A. et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 26, 2435–2444 (2007).
Ren, X. et al. Identification of Niclosamide as a New Small-Molecule Inhibitor of the STAT3 Signaling Pathway. ACS Med. Chem. Lett. 1, 454–459 (2010).
Zhou, T. H. et al. Establishment and optimization of co-culture technology for breast cancer organoids. J. Shanghai Jiao Tong Univ. (Med. Sci.) 41, 1017–1024 (2021).
Sardi, S. P., Murtie, J., Koirala, S., Patten, B. A. & Corfas, G. Presenilin-dependent ErbB4 nuclear signaling regulates the timing of astrogenesis in the developing brain. Cell 127, 185–197 (2006).
Wang, S. C. & Hung, M. C. Nuclear translocation of the epidermal growth factor receptor family membrane tyrosine kinase receptors. Clin. Cancer Res. 15, 6484–6489 (2009).
Aleksic, T. et al. Nuclear IGF1R interacts with regulatory regions of chromatin to promote RNA polymerase II recruitment and gene expression associated with advanced tumor stage. Cancer Res. 78, 3497–3509 (2018).
Asmane, I. et al. Insulin-like growth factor type 1 receptor (IGF-1R) exclusive nuclear staining: a predictive biomarker for IGF-1R monoclonal antibody (Ab) therapy in sarcomas. Eur. J. Cancer 48, 3027–3035 (2012).
Liccardi, G., Hartley, J. A. & Hochhauser, D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 71, 1103–1114 (2011).
Lo, H. W. et al. Novel prognostic value of nuclear epidermal growth factor receptor in breast cancer. Cancer Res. 65, 338–348 (2005).
Lo, H. W. & Hung, M. C. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br. J. Cancer 94, 184–188 (2006).
Psyrri, A. et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clin. Cancer Res. 11, 5856–5862 (2005).
Reilly, J. F. & Maher, P. A. Importin beta-mediated nuclear import of fibroblast growth factor receptor: role in cell proliferation. J. Cell Biol. 152, 1307–1312 (2001).
Giri, D. K. et al. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol. Cell Biol. 25, 11005–11018 (2005).
Wang, Y. N. et al. The translocon Sec61beta localized in the inner nuclear membrane transports membrane-embedded EGF receptor to the nucleus. J. Biol. Chem. 285, 38720–38729 (2010).
Stachowiak, M. K., Maher, P. A. & Stachowiak, E. K. Integrative nuclear signaling in cell development-a role for FGF receptor-1. DNA Cell Biol. 26, 811–826 (2007).
Liao, H. J. & Carpenter, G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol. Biol. Cell 18, 1064–1072 (2007).
He, W. et al. IL22RA1/STAT3 signaling promotes stemness and tumorigenicity in pancreatic cancer. Cancer Res. 78, 3293–3305 (2018).
Rokavec, M. et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Invest. 124, 1853–1867 (2014).
Lin, W. H. et al. STAT3 phosphorylation at Ser727 and Tyr705 differentially regulates the EMT-MET switch and cancer metastasis. Oncogene 40, 791–805 (2021).
Niit, M. et al. Cell-cell and cell-matrix adhesion in survival and metastasis: Stat3 versus Akt. Biomol. Concepts. 6, 383–399 (2015).
Dees, C. et al. TGF-beta-induced epigenetic deregulation of SOCS3 facilitates STAT3 signaling to promote fibrosis. J. Clin. Invest. 130, 2347–2363 (2020).
Green, M. D. et al. Gefitinib treatment in hormone-resistant and hormone receptor-negative advanced breast cancer. Ann. Oncol. 20, 1813–1817 (2009).
Dickler, M. N. et al. A phase II trial of erlotinib in combination with bevacizumab in patients with metastatic breast cancer. Clin. Cancer Res. 14, 7878–7883 (2008).
Thomas, M. B. et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J. Clin. Oncol. 27, 843–850 (2009).
von Minckwitz, G. et al. A multicentre phase II study on gefitinib in taxane- and anthracycline-pretreated metastatic breast cancer. Breast Cancer Res. Treat. 89, 165–172 (2005).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
van den Bent, M. J. et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J. Clin. Oncol. 27, 1268–1274 (2009).
Reardon, D. A. et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. J. Neurooncol. 96, 219–230 (2010).
Brand, T. M., Iida, M. & Wheeler, D. L. Molecular mechanisms of resistance to the EGFR monoclonal antibody cetuximab. Cancer Biol. Ther. 11, 777–792 (2011).
Case, L. B., Ditlev, J. A. & Rosen, M. K. Regulation of transmembrane signaling by phase separation. Annu. Rev. Biophys. 48, 465–494 (2019).
Arkhipov, A. et al. Architecture and membrane interactions of the EGF receptor. Cell 152, 557–569 (2013).
Chen, D. J. & Nirodi, C. S. The epidermal growth factor receptor: a role in repair of radiation-induced DNA damage. Clin Cancer Res. 13, 6555–6560 (2007).
Chen, H., Chen, X. & Zheng, Y. The nuclear lamina regulates germline stem cell niche organization via modulation of EGFR signaling. Cell Stem Cell 13, 73–86 (2013).
Bryant, D. M. & Stow, J. L. Nuclear translocation of cell-surface receptors: lessons from fibroblast growth factor. Traffic 6, 947–954 (2005).
Wells, A. & Marti, U. Signalling shortcuts: cell-surface receptors in the nucleus? Nat. Rev. Mol. Cell Biol. 3, 697–702 (2002).
Massie, C. & Mills, I. G. The developing role of receptors and adaptors. Nat. Rev. Cancer 6, 403–409 (2006).
Wang, S. C. et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nat. Cell Biol. 8, 1359–1368 (2006).
An, L. et al. Dual-utility NLS drives RNF169-dependent DNA damage responses. Proc. Natl. Acad. Sci. USA 114, E2872–E2881 (2017).
Wal, M. & Pugh, B. F. Genome-wide mapping of nucleosome positions in yeast using high-resolution MNase ChIP-Seq. Methods Enzymol. 513, 233–250 (2012).
Meng, Y. et al. A TNFR2-hnRNPK Axis Promotes Primary Liver Cancer Development via Activation of YAP Signaling in Hepatic Progenitor Cells. Cancer Res. 81, 3036–3050 (2021).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82073269, 81772802, M-0349, 32070710, 81902806 and 82002820), Shanghai Science and Technology Innovation Action Plan (20XD1402800), Clinical Research Plan of SHDC (SHDC2020CR2065B), Clinical Research Innovation Plan of Shanghai General Hospital (CTCCR-2016B05), the Shanghai Pujiang Program (19PJ1408300), the Open Research Fund of State Key Laboratory of Genetic Engineering, Fudan University (SKLGE-2103), Natural Science Foundation of Jiangsu Province (BK20181186) and Shanghai Science and Technology Commission (20JC1410100).
Author information
Authors and Affiliations
Contributions
H.W., X.L. and L.A. designed research. X.L., L.A., G.F. and L.Z performed most cellular and in vitro experiments with input from W.H., J.Li., and Y.C.; J.Liu., W.G., J.X. G.F., S.D, T.Z. and H.Y carried out the PDO assay. Y.H. and L.Y. helped imaging analysis and quantification. L.A. and S.J. analyzed the IHC data. All authors discussed and commented on the manuscript. H.W., L.A. and G.F. wrote and revised the paper. H.W. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Lu, X., An, L., Fan, G. et al. EGFR signaling promotes nuclear translocation of plasma membrane protein TSPAN8 to enhance tumor progression via STAT3-mediated transcription. Cell Res 32, 359–374 (2022). https://doi.org/10.1038/s41422-022-00628-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41422-022-00628-8
This article is cited by
-
Extracellular vesicles: the “Trojan Horse” within breast cancer host microenvironments
Molecular Cancer (2025)
-
Cancer stem cells-derived exosomal TSPAN8 enhances non-stem cancer cells stemness and promotes malignant progression in PDAC
Oncogene (2025)
-
Activation of kappa opioid receptor suppresses post-traumatic osteoarthritis via sequestering STAT3 on the plasma membrane
Cell Communication and Signaling (2024)
-
Diagnostic and prognostic significance of tetraspanin 6 and its role in facilitating glioma progression
European Journal of Medical Research (2024)
-
Perturbation of mammary epithelial cell apicobasal polarity by RHBDF1-facilitated nuclear translocation of PKCζ
Biological Research (2024)