Abstract
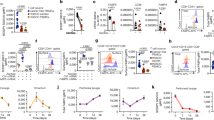
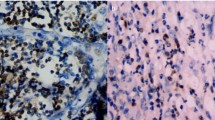
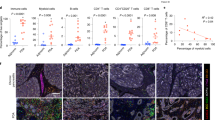
Recruitment of polymorphonuclear MDSCs (PMN-MDSCs) in the TME suppresses the antitumor activity of tumor-infiltrating CD8+ T cells (CD8+ TILs). Little is known about the role of antitumoral CD8+ TILs in actively initiating an immune-tolerant microenvironment, particularly in the recruitment of PMN-MDSCs. In this study, we found that immunotherapy-activated CD8+ TILs significantly increased PNM-MDSC infiltration in the TME, resulting in antitumor resistance. When CD8+ T cells are activated, lipocalin-2 (LCN2) expression is strongly upregulated, which significantly enhances PMN-MDSC chemotaxis. Mechanistically, immune activation increased fatty acid synthesis in CD8+ T cells, particularly oleic acid (OA), which induced lysosomal membrane permeabilization, releasing cathepsin B and subsequently activating NF-κB to promote LCN2 expression. Moreover, we showed that glucagon-like peptide 1 (GLP1) effectively inhibited OA synthesis in activated CD8+ T cells, reducing LCN2 production. We then developed a recombinant adenovirus encoding GLP1 (AdV-GLP1), which significantly reduced PMN-MDSC infiltration and reinvigorated the antitumor activity of CD8+ TILs. In various pancreatic cancer models, including subcutaneous, orthotopic, and humanized CDX/PDX models, AdV-GLP1 displayed excellent antitumor efficacy. Our work advances the understanding of how immunotherapy-activated CD8+ TILs initiate PMN-MDSC infiltration and provides a clinically relevant strategy to target this interaction and improve cancer immunotherapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schenkel JM, Pauken KE. Localization, tissue biology and T cell state—Implications for cancer immunotherapy. Nat. Rev. Immunol. 2023;23:807–23.
Koch M, Beckhove P, op den Winkel J, Autenrieth D, Wagner P, Nummer D, et al. Tumor infiltrating T lymphocytes in colorectal cancer: Tumor-selective activation and cytotoxic activity in situ. Ann. Surg. 2006;244:986–93.
Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018;19:108–19.
Giles JR, Globig A-M, Kaech SM, Wherry EJ. CD8+ T cells in the cancer-immunity cycle. Immunity. 2023;56:2231–53.
Raskov H, Orhan A, Gaggar S, Gögenur I. Neutrophils and polymorphonuclear myeloid-derived suppressor cells: an emerging battleground in cancer therapy. Oncogenesis. 2022;11:22.
Wang C, Zheng X, Zhang J, Jiang X, Wang J, Li Y, et al. CD300ld on neutrophils is required for tumor-driven immune suppression. Nature. 2023;621:830–9.
Highfill SL, Cui Y, Giles AJ, Smith JP, Zhang H, Morse E, et al. Disruption of CXCR2-mediated MDSC tumor trafficking enhances anti-PD1 efficacy. Sci. Transl. Med. 2014;6:237ra67–ra67.
Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–82.
Reina-Campos M, Scharping NE, Goldrath AW. CD8+ T-cell metabolism in infection and cancer. Nat. Rev. Immunol. 2021;21:718–38.
Wang R, Liu Z, Fan Z, Zhan H. Lipid metabolism reprogramming of CD8+ T cell and therapeutic implications in cancer. Cancer Lett. 2023;567:216267.
Lim SA, Su W, Chapman NM, Chi H. Lipid metabolism in T cell signaling and function. Nat Chem Biol. 2022;18:470–81.
Ma X, Xiao L, Liu L, Ye L, Su P, Bi E, et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T-cell effector function and impairs their antitumor ability. Cell Metab. 2021;33:1001–12.e5.
Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, et al. STAT3 activation-induced fatty acid oxidation in CD8+ T effector cells is critical for obesity-promoted breast tumor growth. Cell Metab. 2020;31:148–61.e5.
Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR-induced fatty acid oxidation in T cells increases the number of tumor-reactive CD8+ T cells and facilitates anti–PD-1 therapy. Cancer Immunol. Res. 2018;6:1375–87.
Lin R, Zhang H, Yuan Y, He Q, Zhou J, Li S, et al. Fatty acid oxidation controls CD8+ tissue-resident memory T-cell survival in gastric adenocarcinoma. Cancer Immunol. Res. 2020;8:479–92.
Manzo T, Prentice BM, Anderson KG, Raman A, Schalck A, Codreanu GS, et al. Accumulation of long-chain fatty acids in the tumor microenvironment drives dysfunction in intrapancreatic CD8+ T cells. J Exp Med. 2020;217:e20191920.
Lauson CBN, Tiberti S, Corsetto PA, Conte F, Tyagi P, Machwirth M, et al. Linoleic acid potentiates CD8+ T-cell metabolic fitness and antitumor immunity. Cell Metab. 2023;35:633–50.e9.
Sun S, Xu H, Zhao W, Li Q, Yuan Y, Zhang G, et al. PA suppresses antitumor immunity of T cells by disturbing mitochondrial activity through Akt/mTOR-mediated Ca2+ flux. Cancer Lett. 2024;581:216511.
Sugi T, Katoh Y, Ikeda T, Seta D, Iwata T, Nishio H, et al. SCD1 inhibition enhances the effector functions of CD8+ T cells via ACAT1‐dependent reduction of esterified cholesterol. Cancer Sci. 2024;115:48–58.
Shalhout SZ, Miller DM, Emerick KS, Kaufman HL. Therapy with oncolytic viruses: progress and challenges. Nat. Rev. Clin. Oncol. 2023;20:160–77.
Gujar S, Pol JG, Kumar V, Lizarralde-Guerrero M, Konda P, Kroemer G, et al. Tutorial: design, production and testing of oncolytic viruses for cancer immunotherapy. Nat Protoc. 2024;19:2540–70.
Watanabe M, Nishikawaji Y, Kawakami H, Kosai K. Adenovirus biology, recombinant adenovirus, and adenovirus usage in gene therapy. Viruses. 2021;13:2502.
Ullman NA, Burchard PR, Dunne RF, Linehan DC. Immunologic strategies in pancreatic cancer: making cold tumors hot. J. Clin. Oncol. 2022;40:2789–805.
Royal RE, Levy C, Turner K, Mathur A, Hughes M, Kammula US, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010;33:828–33.
Shao S, Cao T, Jin L, Li B, Fang H, Zhang J, et al. Increased lipocalin-2 contributes to the pathogenesis of psoriasis by modulating neutrophil chemotaxis and cytokine secretion. J. Investigative Dermatol. 2016;136:1418–28.
Wieser V, Tymoszuk P, Adolph TE, Grander C, Grabherr F, Enrich B, et al. Lipocalin 2 drives neutrophilic inflammation in alcoholic liver disease. J. Hepatol. 2016;64:872–80.
Kinoshita M, Ogawa Y, Hama N, Ujiie I, Hasegawa A, Nakajima S, et al. Neutrophils initiate and exacerbate Stevens-Johnson syndrome and toxic epidermal necrolysis. Sci. Transl. Med. 2021;13:eaax2398.
Kovács SA, Fekete JT, Győrffy B. Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors. Acta Pharmacologica Sin. 2023;44:1879–89.
Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid–induced hepatic lipotoxicity. Hepatology. 2008;47:1495–503.
Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF‐α expression via a lysosomal pathway. Hepatology. 2004;40:185–94.
Schwartz EA, Koska J, Mullin MP, Syoufi I, Schwenke DC, Reaven PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis. 2010;212:217–22.
Matikainen N, Mänttäri S, Schweizer A, Ulvestad A, Mills D, Dunning BE, et al. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49:2049–57.
Ding X, Saxena NK, Lin S, Gupta N, Anania FA. Exendin‐4, a glucagon‐like protein‐1 (GLP‐1) receptor agonist, reverses hepatic steatosis in ob/ob mice. Hepatology. 2006;43:173–81.
Chen J, Zhao H, Ma X, Zhang Y, Lu S, Wang Y, et al. GLP-1/GLP-1R signaling in regulation of adipocyte differentiation and lipogenesis. Cell. Physiol. Biochem. 2017;42:1165–76.
Chu Y, Dai E, Li Y, Han G, Pei G, Ingram DR, et al. Pancancer T-cell atlas links a cellular stress response state to immunotherapy resistance. Nat. Med. 2023;29:1550–62.
Bailey P, Chang DK, Nones K, Johns AL, Patch A-M, Gingras M-C, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52.
Witkiewicz AK, McMillan EA, Balaji U, Baek G, Lin W-C, Mansour J, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 2015;6:6744.
Pham TN, Shields MA, Spaulding C, Principe DR, Li B, Underwood PW, et al. Preclinical models of pancreatic ductal adenocarcinoma and their utility in immunotherapy studies. Cancers. 2021;13:440.
Jia Y, Wang Y, Dunmall LSC, Lemoine NR, Wang P, Wang Y. Syrian hamster as an ideal animal model for evaluation of cancer immunotherapy. Front. Immunol. 2023;14:1126969.
Tysome JR, Li X, Wang S, Wang P, Gao D, Du P, et al. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin. Cancer Res. 2012;18:6679–89.
Wang J-L, Quan Q, Ji R, Guo X-Y, Zhang J-M, Li X, et al. Isorhamnetin suppresses PANC-1 pancreatic cancer cell proliferation through S phase arrest. Biomedicine Pharmacother. 2018;108:925–33.
Hidalgo M, Amant F, Biankin AV, Budinská E, Byrne AT, Caldas C, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4:998–1013.
Garcia PL, Miller AL, Yoon KJ. Patient-derived xenograft models of pancreatic cancer: overview and comparison with other types of models. Cancers. 2020;12:1327.
Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor‐induced tolerance and immune suppression by myeloid derived suppressor cells. Immunological Rev. 2008;222:162–79.
Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Investig. 2015;125:3356–64.
Sade-Feldman M, Kanterman J, Klieger Y, Ish-Shalom E, Olga M, Saragovi A, et al. Clinical significance of circulating CD33+ CD11b+ HLA-DR− myeloid cells in patients with stage IV melanoma treated with ipilimumab. Clin. Cancer Res. 2016;22:5661–72.
Weber J, Gibney G, Kudchadkar R, Yu B, Cheng P, Martinez AJ, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol. Res. 2016;4:345–53.
Asaf S, Maqsood F, Jalil J, Sarfraz Z, Sarfraz A, Mustafa S, et al. Lipocalin 2—not only a biomarker: a study of current literature and systematic findings of ongoing clinical trials. Immunologic Res. 2023;71:287–313.
Liu C, Cai Z, Hu T, Yao Q, Zhang L. Cathepsin B aggravated doxorubicin‑induced myocardial injury via NF‑κB signaling. Mol. Med. Rep. 2020;22:4848–56.
Yao F, Deng Y, Zhao Y, Mei Y, Zhang Y, Liu X, et al. A targetable LIFR− NF-κB− LCN2 axis controls liver tumorigenesis and vulnerability to ferroptosis. Nat. Commun. 2021;12:7333.
Lin L, Hu M, Li Q, Du L, Lin L, Xue Y, et al. Oleic acid availability impacts thymocyte preprogramming and subsequent peripheral Treg cell differentiation. Nat. Immunol. 2024;25:54–65.
Bruen R, Curley S, Kajani S, Crean D, O’Reilly ME, Lucitt MB, et al. Liraglutide dictates macrophage phenotype in apolipoprotein E null mice during early atherosclerosis. Cardiovascular Diabetol. 2017;16:1–13.
Huang J, Yi H, Zhao C, Zhang Y, Zhu L, Liu B, et al. Glucagon-like peptide-1 receptor (GLP-1R) signaling ameliorates dysfunctional immunity in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3191–202.
Wong CK, Yusta B, Koehler JA, Baggio LL, McLean BA, Matthews D, et al. Divergent roles for the gut intraepithelial lymphocyte GLP-1R in control of metabolism, microbiota, and T-cell-induced inflammation. Cell Metab. 2022;34:1514–31. e7.
Bendotti G, Montefusco L, Lunati ME, Usuelli V, Pastore I, Lazzaroni E, et al. The anti-inflammatory and immunological properties of GLP-1 Receptor Agonists. Pharmacol. Res. 2022;182:106320.
Mathewson ND, Ashenberg O, Tirosh I, Gritsch S, Perez EM, Marx S, et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell. 2021;184:1281–98.e26.
Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21.
Hu Y. Isolation of human and mouse neutrophils ex vivo and in vitro. Methods. Mol Biol. 2012;844:101–13.
Jo EJ, Bae E, Yoon J-H, Kim JY, Han JS. Comparison of murine retroorbital plexus and facial vein blood collection to mitigate animal ethics issues. Lab. Anim. Res. 2021;37:12.
Wang S, Yan W, Kong L, Zuo S, Wu J, Zhu C, et al. Oncolytic viruses engineered to enforce cholesterol efflux restore tumor-associated macrophage phagocytosis and anti-tumor immunity in glioblastoma. Nat Commun. 2023;14:4367
Ma T, Lu W, Wang Y, Qian P, Tian H, Gao X, et al. An oral GLP-1 and GIP dual receptor agonist improves metabolic disorders in high fat-fed mice. Eur. J. Pharmacol. 2022;914:174635.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (82273261 to JW, 82073367 to MX), Nanjing University (0214/151130 to JW) and the State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University (ZZYJ-202401 to JW). We are grateful to TissueGnostics Asia Pacific Ltd. for their technical support. We thank Dr. Hua Zhang (SPH Biotherapeutics (HK) Limited) for kindly providing the HPD-1NR cell line.
Author information
Authors and Affiliations
Contributions
JW and JD conceived the study, designed the experiments, and supervised the project. JW, PQ, YH, CX, and JD performed the experiments. JW, JD, PQ, MX, PZ, and JW analyzed the data. JW, JD, and JW wrote the original draft of the paper. All the authors critically reviewed and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, J., Qian, P., Han, Y. et al. GLP1 alleviates oleic acid-propelled lipocalin-2 generation by tumor-infiltrating CD8+ T cells to reduce polymorphonuclear MDSC recruitment and enhances viral immunotherapy in pancreatic cancer. Cell Mol Immunol 22, 282–299 (2025). https://doi.org/10.1038/s41423-025-01260-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-025-01260-3
Keywords
This article is cited by
-
NF-κB in inflammation and cancer
Cellular & Molecular Immunology (2025)